Stem Cell Therapy Market Size Projections and Insights, 2031
The global stem cell therapy market size was valued at $205.1 million in 2021, and is projected to reach $928.6 million by 2031, growing at a CAGR of 16.2% from 2022 to 2031. The Asia-Pacific Stem Cell Therapy market is significantly impacted by the growing pharmaceutical and biotechnology industry in the region. Adipose tissue-derived mesenchymal stem cells are widely adopted for the treatment of cancer. By type cell therapy has dominated the market share and is expected to remain dominant during the forecast period.
Stem Cell Therapy Market Overview & Growth Drivers
Stem cell therapy is a cutting-edge medical treatment that uses stem cells to repair or replace damaged tissues and organs. By harnessing the unique ability of stem cells to develop into various cell types, this therapy holds promise for treating a range of conditions, from neurodegenerative diseases to injuries. Researchers are exploring its potential to regenerate damaged tissues, enhance healing, and even tackle chronic diseases.
The global stem cell therapy market growth is driven by increasing prevalence of chronic diseases, advancements in stem cell research, and the growing demand for regenerative medicine. According to 2023 article by National Cance Institute, stem cell transplants are most often used to treat people with cancers that affect blood cells, such as leukemia, lymphoma, multiple myeloma, and myelodysplastic syndromes. The rise in chronic conditions like cardiovascular diseases, diabetes, and neurodegenerative disorders fuels the need for innovative treatment options, where stem cell therapy shows significant promise.
Additionally, ongoing research and development in stem cell technology are enhancing the efficacy and safety of treatments, further propelling market growth. The growing acceptance of regenerative medicine, coupled with supportive government initiatives and investments, also contributes to market expansion. Moreover, the increasing number of clinical trials and the rising focus on personalized medicine are expected to drive the adoption of stem cell therapies. However, the market faces challenges such as high treatment costs, ethical concerns, and regulatory hurdles, which may impact its growth trajectory. Overall, while these driving factors create a favorable environment for market growth, addressing the associated challenges is crucial for sustained advancement in the stem cell therapy sector.
Industry Highlights
- Significant increase in funding and investments from both private and public sectors is driving the expansion of the stem cell therapy market. This surge is attributed to the promising potential of stem cell treatments in addressing a wide range of medical conditions.
- Rapid advancements in stem cell research and technology are contributing to the development of innovative therapies. Breakthroughs in gene editing, cell reprogramming, and regenerative medicine are expanding the therapeutic applications and efficacy of stem cell treatments.
- The demand for stem cell therapies is rising due to the increasing prevalence of chronic diseases and conditions such as cancer, neurodegenerative disorders, and cardiovascular diseases
- Improved regulatory frameworks and approval processes are facilitating market growth. As more stem cell therapies receive regulatory approvals, their availability in the market is increasing, which is contributing to the overall market expansion and accessibility for patients.
Advances in research and development (R&D) have profoundly impacted the stem cell therapy industry, driving its growth and expanding its potential. In February 2024, the first CRISPR/Cas9 gene-edited therapy has been granted a marketing authorization by the European Commission (EC). Vertex Pharmaceuticals’ CASGEVY is conditionally approved individuals 12 years and over with severe sickle cell disease characterized by recurrent vaso-occlusive crises or transfusion-dependent beta thalassemia. Innovations in stem cell technology, such as improved isolation and culture techniques, have enhanced the efficacy and safety of therapies. Breakthroughs in gene editing, including CRISPR-Cas9, allow for precise modifications of stem cells, which can correct genetic defects and tailor treatments to individual patients. Enhanced understanding of stem cell biology and differentiation pathways has led to the development of more targeted and effective therapies for a range of conditions, from neurodegenerative diseases to cardiovascular disorders. Additionally, advancements in tissue engineering and regenerative medicine are creating new opportunities for stem cell applications in organ repair and transplantation the stem cell therapy market, positioning it for continued expansion and improved patient outcomes.
Key Areas Covered in the Report
- The report highlights the major players in the stem cell therapy market, including pharmaceutical companies, biotech firms, and research institutions. It provides insights into their market share, strategic initiatives such as mergers and acquisitions, partnerships, and product innovations, as well as their positioning within the market.
- The report analyzes the regulatory environment affecting stem cell therapy, including approvals, ethical considerations, and compliance issues.
- The report highlights latest technological innovations in stem cell therapy, such as advancements in stem cell culture techniques, gene editing, and personalized therapies. This section highlights the impact of these innovations on market growth, including the challenges and opportunities they present for both existing and emerging players in the market. It also covers the role of research and development in driving new therapy options and improving treatment efficacy.
- The report evaluates the competitive intensity and attractiveness of the stem cell therapy market trends using Porter's Five Forces model. This includes analyzing the threat of new entrants, bargaining power of suppliers and buyers, threat of substitute products, and the intensity of competitive rivalry. The report provides insights into how these forces shape market dynamics and influence strategic decision-making.
Advancements in gene editing have significantly impacted the stem cell therapy market growth by enhancing the precision and efficacy of treatments. Technologies like CRISPR-Cas9 and TALENs have enabled targeted modifications to the genetic makeup of stem cells, allowing for the correction of genetic defects and the development of more personalized therapies. This has expanded the potential applications of stem cell therapy, particularly in treating genetic disorders, cancers, and degenerative diseases. Enhanced gene editing techniques have also improved the safety profile of stem cell treatments by reducing off-target effects and enhancing the overall reliability of therapies. As a result, the integration of gene editing into stem cell research and clinical applications has accelerated the development of innovative treatments, driving growth in the stem cell therapy market share and fostering advancements in regenerative medicine.
Topics discussed in the report
- Innovations in gene therapy
- Cancer Treatment
- Gene therapy
- CRISPR-Cas9 technology
Segment Overview
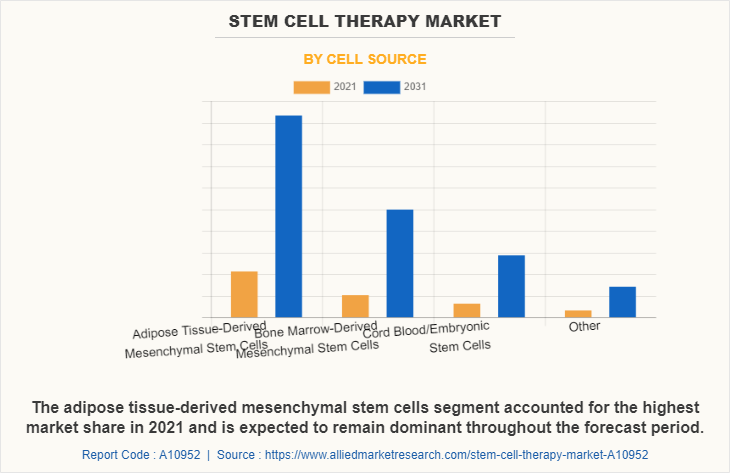
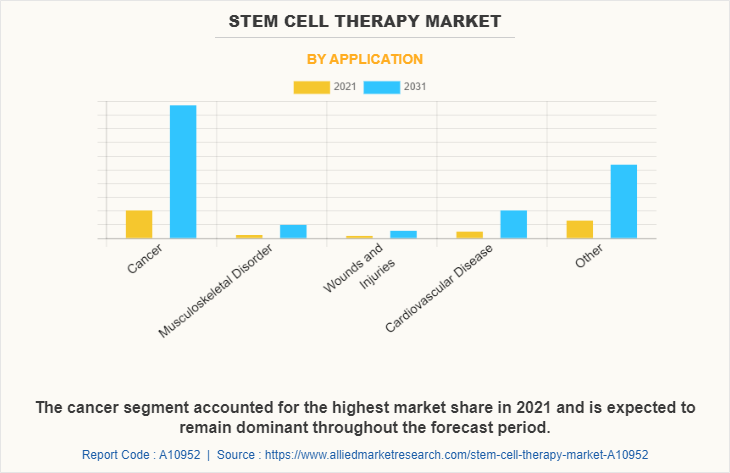
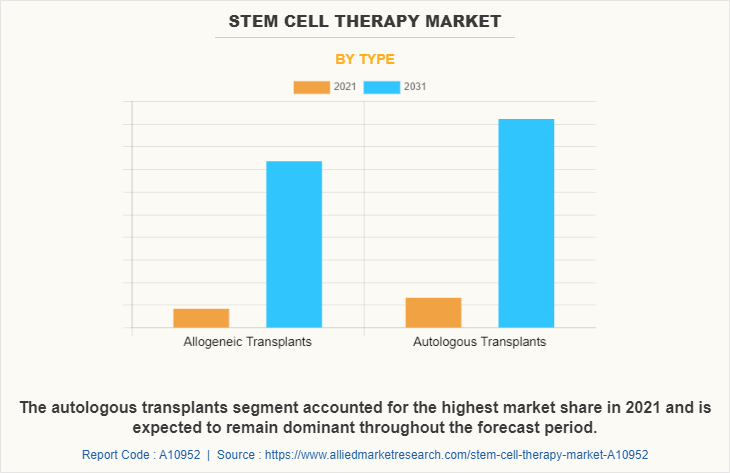
The stem cell therapy market is segmented into cell source, application and type. By cell source, the market is classified into adipose tissue-derived mesenchymal stem cells, bone marrow-derived mesenchymal stem cells, cord blood/embryonic stem cells, and other cell sources. By application, the market is classified into cancer, musculoskeletal disorders, wounds and injuries, cardiovascular diseases, gastrointestinal diseases, and other applications. By type, the market is classified into allogeneic transplants and autologous transplants. Furthermore, allogeneic transplants are sub-classified as pediatric and adult. Also, autologous transplants are sub-classified as pediatric and adult. Region wise, the market is studied across North America (U.S., Canada, and Mexico), Europe (Germany, France, the UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, Australia, India, South Korea, and Rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and Rest of LAMEA).
The adipose tissue-derived mesenchymal stem cells dominated the market share in 2021. This is attributed to the fact that adipose tissue is abundant and can be easily harvested through minimally invasive procedures, making it a more accessible source of stem cells compared to other sources like bone marrow. Furthermore, based on application cancer segment dominated the market share in 2021, owing to growing prevalence of cancer and high adoption of stem cell therapy in cancer treatment. By type, the autologous transplants segment is anticipated to grow with the largest stem cell therapy market share throughout the forecast period. This is attributed to the increase in the use of autologous stem cells, and availability of a number of stem cell products. Recent advances in cellular technology have contributed to improve the understanding of various disease cells and their metabolism at molecular level, thus driving the need of stem cell therapies for the treatment.
Comparative Matrix of Key Segments
Parameters | Adipose Tissue-Derived Mesenchymal Stem Cells | Bone Marrow-Derived Mesenchymal Stem Cells | Cord Blood/Embryonic Stem Cells | Other Cell Sources |
Market Share | Dominated the market share because adipose tissue is relatively easy to obtain through liposuction, and there is a higher concentration of stem cells in adipose tissue compared to bone marrow. | Is expected to register highest CAGR owing to high adoption of bone marrow-derived mesenchymal stem cells in treatments for bone and cartilage repair, as well as for conditions like graft-versus-host disease (GVHD) and other immune disorders. | Is expected to register significant growth owing to the fact that cord blood stem cells have lower risk of immunological rejection, as they are less likely to be rejected by the recipient's immune system. | Other sources like umbilical tissue or dental pulp stem cells offer unique advantages depending on the application, such as ease of collection or specific regenerative properties. |
End User | Orthopedic clinics, cardiology centers, and regenerative medicine specialty center | Hematology clinics, cancer treatment centers, and transplant centers | Hematology and oncology centers, neonatal and pediatric units, and research institutions | Regenerative medicine specialists, research laboratories |
Challenges | Variability in the quality and quantity of ADSCs due to differences in collection and processing methods can affect consistency and efficacy. | The process of obtaining bone marrow-derived mesenchymal stem cells is more invasive and uncomfortable compared to other sources like adipose tissue. | The cost and logistics of cord blood banking and storage can be prohibitive for some families and healthcare systems. | Compared to more established sources, dental pulp stem cells have less clinical data and research backing their efficacy. |
Key Players | Mesoblast Limited, Cytori Therapeutics and Stem Cells, Inc. | Osiris Therapeutics, Athersys, Inc, and Pluristem Therapeutics | Smith & Nephew plc, and Orthofix Holdings, Inc | Dentech, Allele Biotechnology and Pharmaceuticals, Inc., NuVasive, Inc |
Regional Dynamics and Competition
North America has emerged as a major revenue generator in the stem cell therapy industry primarily due to its advanced healthcare infrastructure, high research and development investments, and favorable regulatory environment. The region's robust medical facilities, leading academic institutions, and significant funding for stem cell research contribute to its dominance. Additionally, North America benefits from a high prevalence of chronic diseases and a strong emphasis on innovative treatments, which drive the demand for stem cell therapies.
However, Asia Pacific is expected to register the highest CAGR in the forecast period due to rapid advancements in healthcare infrastructure, increasing investments in research, and a growing patient population with unmet medical needs. The region's expanding middle class, coupled with rising awareness and acceptance of advanced medical technologies, further fuels market growth. Additionally, supportive government initiatives and collaborations between local and international stakeholders are accelerating the development and adoption of stem cell therapies in Asia Pacific.
Some of the major players analyzed in this report are Mesoblast Ltd., Astellas Pharma Inc., Fujifilm Holding Corporation, Novadip Biosciences, U.S. Stem Cell, Inc., Smith & Nephew plc, Takeda Pharmaceutical Company Ltd, Orthofix Holdings, Inc., Allele Biotechnology and Pharmaceuticals, Inc., NuVasive, Inc.
Stem Cell Therapy Market News Release
- In 2021, A worldwide licensed agreement and strategic collaboration between Minovia Therapeutics, Ltd. and Astellas Pharma, Inc. was established for the development of cutting-edge stem cell therapy programs for illnesses brought on by mitochondrial malfunction. The partnership intends to accelerate the development of allogeneic mitochondrial cell therapy initiatives. The two businesses will collaborate to investigate potential cell treatment program prospects using Astellas' genetically modified, induced pluripotent stem cells and Minovia's MAT platform technology.
- In April 2023, Gamida Cell Ltd announced that U.S. Food and Drug Administration approved its Omisirge. Omisirge (omidubicel-onlv) is intended for use in patients 12 years and older with hematologic malignancies scheduled for umbilical cord transplantation following myeloablative conditioning.
- For instance, in March 2022, Wipro Ltd, and Pandorum Technologies, announced a long-term partnership. Together the companies plan to aim on the development of technologies that will reduce the time-to-market and boost the patient outcome during clinical trials and research and development of regenerative medicine.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the Stem Cell Therapy market analysis from 2022 to 2032 to identify the prevailing Stem Cell Therapy market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the Stem Cell Therapy market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global Stem Cell Therapy market trends, key players, market segments, application areas, and market growth strategies.
Stem cell therapy Market Report Highlights
| Aspects | Details |
| Market Size By 2031 | USD 928.6 million |
| Growth Rate | CAGR of 16.2% |
| Forecast period | 2021 - 2031 |
| Report Pages | 274 |
| By Cell Source |
|
| By Application |
|
| By Type |
|
| By Region |
|
Analyst Review
The utilization of stem cell therapy is expected to witness a significant rise owing to the increase in use of stem cell therapy in treatment of diseases such as autoimmune, inflammatory, neurological, orthopedic conditions and traumatic injuries with studies conducted on use for Crohn's disease, multiple sclerosis, lupus, COPD, Parkinson's disease and stroke recovery. Moreover, the prevalence of cancer in both developed and developing economies also aids in market growth.
North America accounted for the highest market share in 2021, due to availability of advance stem cell products, higher cancer awareness, and heavy expenditure by the government on healthcare, followed by Europe and Asia-Pacific. Asia-Pacific is expected to exhibit fastest market growth due to increase in incidence of cancer. Pharmaceutical companies have focused on expanding their presence in emerging economies, which is anticipated to drive the market growth
The estimated industry size of Stem cell therapy market is estimated to be $928.64 million in 2031.
The forecast period in the market report is from 2022 to 2031
The base year calculated in the stem cell therapy market report is 2021
Yes, the stem cell therapy market report provides PORTER Analysis.
The major companies profiled in the report include Allele Biotechnology and Pharmaceuticals, Inc., Astellas Pharma Inc, Fujifilm Holding Corporation (FUJIFILM Cellular Dynamics, Inc.), Mesoblast Ltd, Novadip Biosciences, NuVasive, Inc., Orthofix Holdings, Inc., Smith & Nephew plc, Takeda Pharmaceutical, and U.S. Stem Cell, Inc.
The increase in demand for stem cell therapy, increase in government support for R&D activities and increase in healthcare expenditure are the key trends of stem cell therapy market.
The leading application of stem cell therapy is that it promotes the tissue repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives.
North America is the largest regional market for Stem cell therapy.
Loading Table Of Content...



