Amebocyte Lysate Market Research, 2033
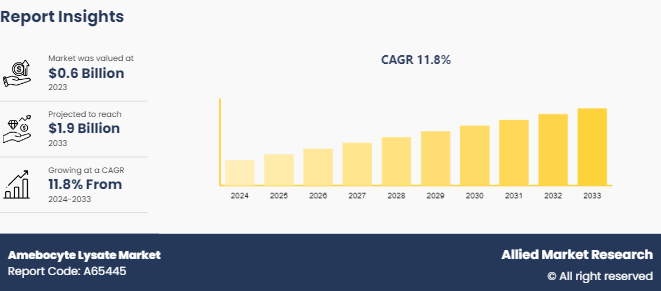
The global amebocyte lysate market size was valued at $0.6 billion in 2023, and is projected to reach $1.9 billion by 2033, growing at a CAGR of 11.8% from 2024 to 2033. The growth of the amebocyte lysate market is primarily driven by increasing pharmaceutical production, stringent regulations for endotoxin detection, and rising demand for rapid pyrogen testing methods in healthcare industries.
Market Introduction and Definition
Amebocyte lysate is a crucial component in the field of biomedical research and pharmaceuticals, particularly in the detection of bacterial endotoxins. Derived from the blood cells, or amebocytes, of horseshoe crabs, this lysate possesses a unique property of clotting in the presence of bacterial endotoxins. The process involves collecting the blood of horseshoe crabs, isolating the amebocytes, and then lysing them to release the lysate. This lysate contains a substance called limulus amebocyte lysate (LAL) , which is sensitive to minute amounts of bacterial endotoxins. When LAL comes into contact with endotoxins, it triggers a biochemical reaction that leads to the formation of a gel-like clot, providing a visible indication of the presence of endotoxins. This method is highly sensitive and widely used in industries such as pharmaceuticals and medical devices to ensure the safety and quality of products, as endotoxins can cause severe adverse reactions in humans. Moreover, due to its remarkable sensitivity, amebocyte lysate has found applications in environmental monitoring, particularly in assessing the quality of water and other environmental samples for bacterial contamination.
Key Takeaways
- The amebocyte lysate market share study covers 20 countries. The research includes a segment analysis of each country in terms of value for the projected period 2024-2032.
- More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major amebocyte lysate industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrates high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and to assist stakeholders in making educated decisions in order to achieve their most ambitious growth objectives.
Key Market Dynamics
Key factors driving the amebocyte lysate market growth are high adoption of amebocyte lysate by pharmaceuticals, biotechnology, and medical devices companies to adhere to strict standards for ensuring product safety. According to 2023 article by the National Library of Medicine, it was reported that LAL assay is routinely used in the pharmaceutical industry to detect endotoxin contamination in crystalloid solutions and pharmacological preparations such as vaccines. Furthermore, increasing focus on quality control is expected to drive the growth of the market. With the emphasis on ensuring the safety and efficacy of pharmaceutical products, there is a growing focus on quality control measures throughout the manufacturing process. Amebocyte lysate-based assays offer rapid and accurate detection of endotoxins, facilitating efficient quality control procedures. Thus, the growing focus on quality control is expected to drive the growth of amebocyte lysate market size.
In addition, according to amebocyte lysate market opportunity analysis increasing focus on maintaining stringent regulatory standards in pharmaceutical industry has positively impacted the demand for amebocyte lysate. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) mandate rigorous testing of pharmaceutical products for endotoxin contamination. Compliance with these standards fuels the demand for reliable and sensitive endotoxin detection methods, thus driving the market for amebocyte lysate.
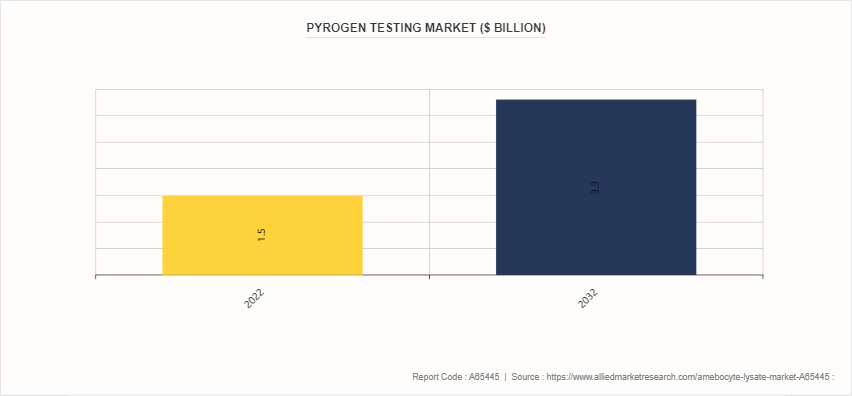
Parent Market Overview - Pyrogen Testing Market
Pyrogen testing market is parent market for amebocyte lysate market. The pyrogen testing market encompasses a range of methods and products utilized to ensure the safety of pharmaceuticals, medical devices, and other healthcare products by detecting and quantifying pyrogenic contaminants. Pyrogens, such as endotoxins from bacterial cell walls or non-endotoxin substances, can induce fever and other potentially harmful immune responses when introduced into the body. One critical component in pyrogen testing is the use of amebocyte lysate, derived from the blood cells of horseshoe crabs. Amebocyte lysate serves as a sensitive tool for detecting the presence of endotoxins, which are a common type of pyrogen. The speed, sensitivity, reliability of amebocyte lysate make them indispensable in ensuring the safety of injectable drugs, intravenous fluids, and implantable medical devices.
Market Segmentation
The global amebocyte lysate market is segmented into type, application, and region. By type, the market is divided into limulus amebocyte lysate and tachypleus amebocyte lysate. On the basis of application, it is segregated into drug testing, clinical diagnosis, and other. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional/Country Market Outlook
The amebocyte lysate market exhibits a diverse regional outlook driven by factors such as regulatory landscape, healthcare infrastructure, and industry dynamics. North America dominated the amebocyte lysate market share in 2023. This is attributed to well-developed pharmaceutical industry and stringent regulatory standard to ensure high quality of pharmaceutical products propel the market growth. Europe follows a similar trajectory, with robust regulatory frameworks such as those established by the European Pharmacopoeia driving demand for amebocyte lysate-based testing methods. However, according to amebocyte lysate market forecast analysis Asia-Pacific is expected to emerges as a lucrative market propelled by rapid industrialization, expanding healthcare infrastructure, and increasing investments in pharmaceutical manufacturing. The countries such as China and India witness notable growth rates, supported by initiatives to enhance drug quality and safety. Moreover, rising healthcare expenditure and the prevalence of infectious diseases in the region bolster the market expansion. In Latin America and the Middle East & Africa, improving healthcare standards, coupled with efforts to strengthen regulatory compliance, contribute to gradual market growth.
- According to Startup India (a flagship initiative of the Government of India) , it was reported that the pharmaceutical industry in India is expected to reach $65 billion by 2024 and to $130 billion by 2030.
- According to 2023 report by European Federation of Pharmaceutical Industries and Associations, it was reported that in 2022 North America accounted for 52.3% of world pharmaceutical sales compared with. 22.4% for Europe.
Industry Trends
According to 2021 report by the National Library of Medicine, U.S. Pharmacopeia (USP) , Japanese Pharmacopeia (JP) , and European Pharmacopeia (Ph. Eur.) harmonized three types of Limulus Amebocyte Lysate tests (gel-clot, chromogenic, and turbidimetric techniques) for the purpose of evaluating endotoxin contamination in parenteral drugs, medical devices, and raw materials.
According to 2021 report by the U.S. National Science Foundation, it was reported that an estimated 70 million endotoxin tests are performed each year in the U.S. alone.
According to 2021 article by the National Library of Medicine, amebocyte lysate test is the most sensitive and reliable method applied for in vitro detection of bacterial endotoxins.
Competitive Landscape
The major players operating in the amebocyte lysate industry include Lonza Group AG, Charles River Laboratories, Associates Of Cape Cod, Xiamen Bioendo Technology, Zhanjiang A&c Biological, GenScript, Nelson Laboratories, LLC, Thermo Fisher Scientific, Inc., Merck & Co, and Microcoat Biotechnologie GmbH. Other players in amebocyte lysate market include Fuzhou Xinbei, and Wako Chemicals
Recent Key Strategies and Developments
- In March 2024, AmeboGenesis announced a major achievement in sustainable Amebocyte production. The company introduced an innovative technology that allows for the production of bio-identical amebocytes required for LAL production for medical endotoxin testing without the need to harvest horseshoe crab blood. Horseshoe crab blood is crucial for the production of LAL, which is essential for ensuring the safety of injectable drugs, vaccines, and implantable devices globally. This new technology represents a significant advancement in sustainability and has the potential to revolutionize the way LAL is produced.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the amebocyte lysate market analysis from 2024 to 2033 to identify the prevailing amebocyte lysate market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the amebocyte lysate market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global amebocyte lysate market trends, key players, market segments, application areas, and market growth strategies.
Key Sources Referred
- World Health Organization
- Ministry of Health, India
- Center of Disease Control and Prevention.
- National Library of Medicine
- India Investment Grid
- Federation of Pharmaceutical Industries and Associations
Amebocyte Lysate Market Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 1.9 Billion |
| Growth Rate | CAGR of 11.8% |
| Forecast period | 2024 - 2033 |
| Report Pages | 280 |
| By Type |
|
| By Application |
|
| By Region |
|
| Key Market Players | Thermo Fisher Scientific, Inc., Associates of Cape Cod Inc, Zhanjiang A&C Biological, Nelson Laboratories, LLC, Xiamen Bioendo Technology, Charles River Laboratories, Merck & Co., Inc., Genscript Technology Corporation, Lonza Group AG, Microcoat Biotechnologie GmbH |
Analyst Review
The market for amebocyte lysate is witnessing significant growth due to increasing emphasis on product safety and regulatory compliance, particularly in pharmaceutical manufacturing. Moreover, technological advancements and innovations in testing methodologies are further contribute in market growth, enhancing the efficiency and accuracy of endotoxin detection processes.As global health standards continue to evolve and become more rigorous, the importance of amebocyte lysate in ensuring product safety and quality is expected to drive the market growth. However, challenges such as sustainability concerns regarding horseshoe crab populations and the emergence of alternative testing methods might restrain the growth of the market.
The forecast period for Amebocyte Lysate Market is 2024-2033.
The total market value of Amebocyte Lysate Market is $0.6 billion in 2023.
The market value of Amebocyte Lysate Market is projected to reach $1.9 billion by 2033
The base year is 2023 in Amebocyte Lysate Market
Major key players that operate in the Amebocyte Lysate Market are Lonza Group AG, Charles River Laboratories, Associates of Cape Cod, Xiamen Bioendo Technology, and Zhanjiang A&C Biological
Loading Table Of Content...



