Amyotrophic Lateral Sclerosis Treatment Market Research, 2032
The global amyotrophic lateral sclerosis treatment market size was valued at $662.3 million in 2022, and is projected to reach $1,038.94 million by 2032, growing at a CAGR of 4.6% from 2023 to 2032. Amyotrophic lateral sclerosis (ALS) is a devastating disease that affects nerve cells in the brain and spinal cord, leading to muscle weakness and eventually paralysis. The symptoms of amyotrophic lateral sclerosis (ALS) vary from person to person but typically include muscle weakness, stiffness, twitching, difficulty speaking, swallowing, and breathing, and eventually, complete paralysis. The disease progresses at different rates for different people, with some people living for many years with relatively mild symptoms and others experiencing rapid disease progression. There are two main types of amyotrophic lateral sclerosis include sporadic ALS and familial ALS. There are two FDA-approved drugs for the treatment of amyotrophic lateral sclerosis (ALS), which are Riluzole and Edaravone.

“The amyotrophic lateral sclerosis market was decreased during the lockdown period owing to decrease in demand for ALS drugs. In addition, many clinical trials for amyotrophic lateral sclerosis drugs had delayed or postponed. This resulted in a slowdown in the development of new drugs, which ultimately negatively impacted the growth of the market.”
Market Dynamics
Rise in prevalence of amyotrophic lateral sclerosis led to increase in the demand for effective treatments and therapies. For instance, according to an article, ‘Estimated Prevalence and Incidence of Amyotrophic Lateral Sclerosis and SOD1 and C9orf72 Genetic Variants’ published by National Center for Biotechnology Information (NCBI), the ALS prevalence and incidence were 6.22 and 2.31 for Europe. Thus, increase in prevalence of amyotrophic lateral sclerosis led to increase in demand for drugs to treat the disease and further drive the amyotrophic lateral sclerosis treatment market growth.
The growth of the amyotrophic lateral sclerosis treatment market size is expected to be driven by the high potential in untapped emerging markets, due to availability of improved healthcare expenditure, increase in unmet healthcare needs, rise in prevalence of neu, and surge in demand for ALS drugs such as Riluzole.
In addition, increase in awareness among patients about importance of early diagnosis and treatment of amyotrophic lateral sclerosis drives the demand for amyotrophic lateral sclerosis drugs. Rise in product approvals for amyotrophic lateral sclerosis drugs by various key players across the globe is set to affect the market growth positively. For instance, in September 2022, Amylyx Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) approved RELYVRIO (sodium phenylbutyrate and taurursodiol) for the treatment of adults with amyotrophic lateral sclerosis (ALS).
The demand for amyotrophic lateral sclerosis is not only limited to developed countries but is also being witnessed in developing countries, such as China, Brazil, and India, which fuel the growth of the amyotrophic lateral sclerosis treatment market share. Factors such as rise in adoption of ALS drugs and increase in awareness toward amyotrophic lateral sclerosis, further drive the growth of the market.
ALS can be a challenging disease to diagnose, as its symptoms are similar to those of other neurological disorders. The diagnostic process for ALS involves a combination of physical examinations, medical history reviews, and diagnostic tests. Delays in receiving an accurate diagnosis. This delay can impact the effectiveness of treatment and limit the growth of the amyotrophic lateral sclerosis treatment market share. In addition, limited treatment options may limit the growth of the ALS treatment market. Side effects of amyotrophic lateral sclerosis drugs such as diarrhea, nausea, and upper respiratory infection further restrain the market growth.
Furthermore, rise in awareness about ALS may help to increase early diagnosis and treatment. Increased awareness also helps to attract investment in ALS R&D which further supports the market growth. Many government and non-government organizations around the world work toward the treatment and management of amyotrophic lateral sclerosis. Governments have provided funding for research into the causes and treatment of ALS. This funding has supported basic and clinical research to better understand the disease and develop new therapies which support the market growth.
The outbreak of COVID-19 has disrupted workflows in the healthcare sector around the world. The disease has forced a number of industries to shut their doors temporarily, including several sub-domains of health care. The global amyotrophic lateral sclerosis treatment market experienced a decline in 2020 due to global economic recession led by COVID-19. In addition, many hospitals and clinics focused on treating COVID-19 patients, there has been a delay in the diagnosis and treatment of other conditions, including ALS. Patients may be hesitant to seek medical attention during the pandemic or may have difficulty accessing healthcare due to social distancing measures, resulting in delayed diagnosis and treatment which hamper the market growth. Furthermore, many clinical trials for ALS treatments had been disrupted or delayed due to the pandemic. This delays the development of new treatments and slows progress in the field.
However, there is a recovery of the market after the pandemic and stable growth for amyotrophic lateral sclerosis treatment market in the coming future. This is attributed to the increase in adoption of drugs for the treatment of amyotrophic lateral sclerosis and rise in clinical trials for amyotrophic lateral sclerosis.
Segmental Overview
The amyotrophic lateral sclerosis treatment market is segmented into drug, type, distribution channel, and region. On the basis of drug, the market is categorized into Riluzole, Edaravone, and others. On the basis of type, the market is bifurcated into sporadic ALS and familial ALS. On the basis of distribution channel, the market is classified into hospital pharmacies, online providers, and drug stores & retail pharmacies. On the basis of region, the market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, the UK, Italy, Spain, and Rest of Europe), Asia-Pacific (Japan, China, India, Australia, South Korea, and Rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and Rest of LAMEA).
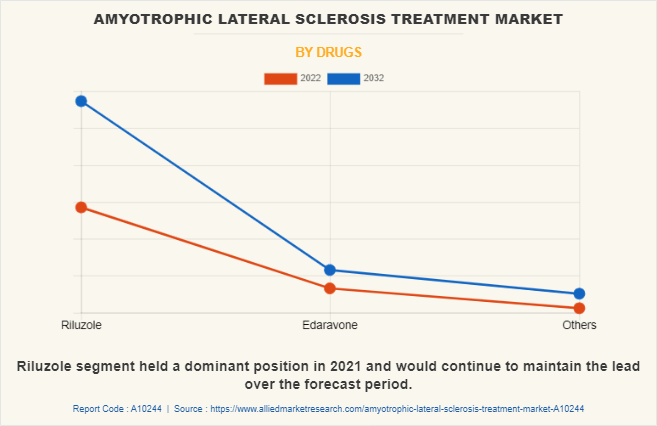
By Drug:
The amyotrophic lateral sclerosis treatment market is segmented into Riluzole, Edaravone, and others.
The riluzole segment dominated the global market in 2022 and is expected to remain dominant throughout the forecast period, owing to increase in adoption of Riluzole for the treatment of amyotrophic lateral sclerosis and the availability of Riluzole by various key players.
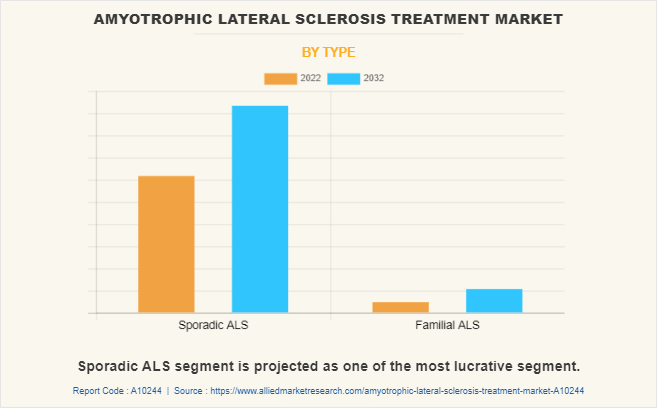
By Type:
The amyotrophic lateral sclerosis treatment market is bifurcated into sporadic ALS and familial ALS. The sporadic ALS segment dominated the global market in 2022 and is anticipated to continue this trend during the forecast period. This is attributed to rise in cases of sporadic amyotrophic lateral sclerosis due to genetic and environmental factors leading to an increase in demand for treatment options.
However, familial ALS segment is expected to register the highest CAGR during the forecast period owing to increase in number of people affected with familial ALS has increased the demand for ALS drugs.
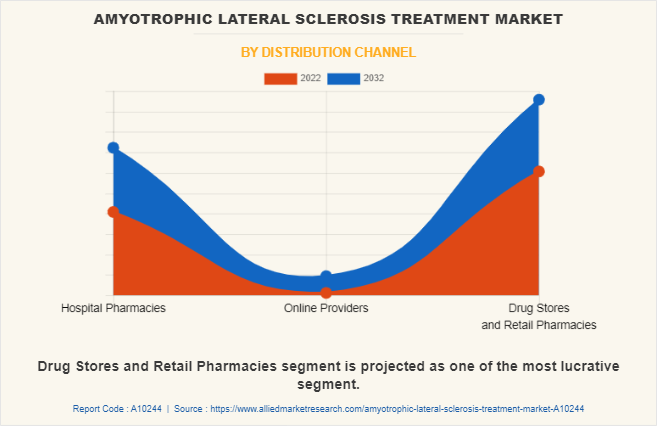
By Distribution Channel:
The amyotrophic lateral sclerosis treatment industry is classified into hospital pharmacies, online providers and drug stores, and retail pharmacies. The drug stores & retail pharmacies segment is the largest market share in 2022 and is expected to remain dominant throughout the forecast period, owing to rise in number of drug stores and retail pharmacies to provide amyotrophic lateral sclerosis drugs. However, online providers segment is expected to register the highest CAGR during the forecast period owing to as they are convenient way for patients to access the drugs and therapies without having to leave their homes.
By Region:
The amyotrophic lateral sclerosis treatment industry is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for a major share of the amyotrophic lateral sclerosis market in 2022 and is expected to maintain its dominance during the forecast period.
In addition, high healthcare expenditure, high purchasing power, and rise in adoption rate of amyotrophic lateral sclerosis drugs are expected to drive the market growth. Moreover, product launches, product approvals, and acquisitions adopted by the key players in this region boost the growth of the market. In addition, there is a higher incidence of ALS in North America due to genetic predisposition, environmental factors, aging population, and a strong focus on R&D.
Asia-Pacific is expected to grow at the highest rate during the forecast period. The market growth in this region is attributable to the presence of pharmaceutical companies in the region as well as growth in the purchasing power of populated countries, such as China and India. Moreover, rise in healthcare expenditure and initiatives taken by government to improve patients life drive the growth of the market.
Furthermore, the Asia-Pacific region exhibits the largest medicine supply and the largest pharmaceuticals industry with availability of raw materials in abundance, which can be easily accessed by manufacturers of amyotrophic lateral sclerosis. This, in turn, drives the growth of the market.
Asia-Pacific offers profitable opportunities for key players operating in the amyotrophic lateral sclerosis market, thereby registering the fastest growth rate during the amyotrophic lateral sclerosis treatment market forecast period, owing to the rise in awareness about early diagnosis and treatment in the region. In addition, growth in aging population as ALS typically affects individuals between the ages of 40 and 70, which provides a great opportunity for new entrants in this region.
Competition Analysis
Competitive analysis and profiles of the major players in amyotrophic lateral sclerosis, such as Aquestive Therapeutics, Inc., Glenmark Pharmaceuticals Limited, Sun Pharmaceutical Industries Ltd., Viatris Inc., Amylyx Pharmaceuticals, Inc., Mitsubishi Chemical Group Corporation, Otsuka Pharmaceutical Co., Ltd., Covis Pharma GmbH, ITALFARMACO S.p.A., and Alkem Laboratories Ltd. are provided in this report. Major players have adopted product launch, product approval, and acquisition as key developmental strategies to improve the product portfolio of the amyotrophic lateral sclerosis treatment market.
Recent Product Launches in the Amyotrophic Lateral Sclerosis Treatment Market
In June 2022, Mitsubishi Tanabe Pharma America, Inc. a subsidiary of Mitsubishi Chemical Holdings Corporation announced that RADICAVA ORS (edaravone) is now available in the U.S. for the treatment of amyotrophic lateral sclerosis (ALS).
Recent Agreement in the Amyotrophic Lateral Sclerosis Treatment Market
In January 2023, C Amylyx Pharmaceuticals, Inc. announced it has entered into an exclusive license and distribution agreement with Neopharm in which Neopharm is expected to commercialize to regulatory review and approval of AMX0035 (sodium phenylbutyrate and ursodoxicoltaurine) for the treatment of amyotrophic lateral sclerosis (ALS) in Israel, Gaza, West Bank, and the Palestinian.
Recent Product Approvals in the Amyotrophic Lateral Sclerosis Treatment Market
In Sepetember 2022, Amylyx Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has approved RELYVRIO (sodium phenylbutyrate and taurursodiol) for the treatment of adults with amyotrophic lateral sclerosis (ALS).
In May 2022, Mitsubishi Tanabe Pharma America, Inc. announced the U.S. Food and Drug Administration (FDA) has approved RADICAVA ORS (edaravone), the oral form of edaravone, for the treatment of amyotrophic lateral sclerosis (ALS).
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the amyotrophic lateral sclerosis treatment market analysis from 2022 to 2032 to identify the prevailing amyotrophic lateral sclerosis treatment market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the amyotrophic lateral sclerosis treatment market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global amyotrophic lateral sclerosis treatment market trends, key players, market segments, application areas, and market growth strategies.
Amyotrophic Lateral Sclerosis Treatment Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 1 billion |
| Growth Rate | CAGR of 4.6% |
| Forecast period | 2022 - 2032 |
| Report Pages | 260 |
| By Drugs |
|
| By Type |
|
| By Distribution Channel |
|
| By Region |
|
| Key Market Players | Aquestive Therapeutics, Inc., Mitsubishi Chemical Group Corporation, Otsuka Pharmaceutical Co., Ltd., Glenmark Pharmaceuticals Limited, Viatris Inc., Alkem Laboratories Ltd, Covis Pharma GmbH, Amylyx Pharmaceuticals, Inc., Sun Pharmaceutical Industries Ltd., ITALFARMACO S.p.A. |
Analyst Review
This section provides various opinions of top-level CXOs in the global amyotrophic lateral sclerosis treatment market. According to the insights of CXOs, the global amyotrophic lateral sclerosis treatment market is expected to exhibit high growth potential attributable to high demand for amyotrophic lateral sclerosis treatment and rise in pipeline products for the treatment of amyotrophic lateral sclerosis. However, side effects of drugs hamper the market growth.?
CXOs further added that rise in number of product approvals for the amyotrophic lateral sclerosis treatment drive the market growth. Moreover, rise in awareness about amyotrophic lateral sclerosis and increase in prevalence of amyotrophic lateral sclerosis are some factors which further boost the market growth.??
Furthermore, North America is expected to witness the largest growth, in terms of revenue, owing to the increase in prevalence of amyotrophic lateral sclerosis in the region. However, Asia-Pacific is anticipated to witness notable growth, owing to rise in healthcare awareness, increase in incidence of amyotrophic lateral sclerosis and easy availability of ALS drugs.
Growth in awareness and understanding of ALS among healthcare professionalsare, innovative drugs for the treatment of amyotrophic lateral sclerosis, key strategies adopted by key players, and increase in awareness regarding treatment of amyotrophic lateral sclerosis are the factors responsible for the market growth.
The leading application of ALS treatment is the use of drugs to manage and treat the symptoms of ALS disease.
North America is the largest regional market for amyotrophic lateral sclerosis owing to early detection, availability of skilled medical professionals, ease of drug availability, and higher adoption of advanced therapeutics.
The estimated industry size of amyotrophic lateral sclerosis in 2032 is $1,038.94 million.
Top companies such as Mitsubishi Chemical Group Corporation, Sun Pharmaceutical Industries Ltd., and Viatris Inc. held high market share in 2022.
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that affects the nerve cells in the brain and spinal cord, leading to the death of the motor neurons that control voluntary muscles.
The forecast period for amyotrophic lateral sclerosis market is 2023 to 2032
Loading Table Of Content...
Loading Research Methodology...


