Real World Evidence Solutions Market Research, 2031
The global Real World Evidence Solutions Market size was valued at $1.4 billion in 2021, and is projected to reach $5 billion by 2031, growing at a CAGR of 13.7% from 2022 to 2031. Real-world evidence is defined as the healthcare data derived from a variety of non-traditional clinical research settings, such as electronic medical records (EMRs), claims and billing information, product and disease registries, and information collected by mobile devices and health apps. The four main sources of collecting real-time data are administrative/claims data, clinical data, patient-generated or reported data and emerging data sources including social media and cross-industry data collaborations such as Project Data Sphere. It is most commonly used by pharmaceutical companies, payors, and providers to better manage their organizations and make decisions about cost-effectiveness and comparative efficacy where other more robust data sources do not exist.

The key factors that drive Real World Evidence Solutions Market growth are increase in the prevalence of cancer and chronic diseases, which increase the demand for the development of novel drugs and medical devices across the world. In addition, rise in adoption of technological advanced real-world evidence solutions; and increase in number of pipeline drugs are the key factors, which further boost the growth of the market. Moreover, rise in R&D activity rates, government initiatives, improved healthcare infrastructure, and increased funding for research projects across the world further propel the growth of the market.
In addition, increase in number of key players and the strategies adopted by them, and rise in demand for technologically advanced medical devices to conduct real time surveys and clinical trials propel the growth of the real-world evidence solutions market.
However, lack of awareness and reluctance to rely on real-world data hampers the market growth. Furthermore, high growth potential in developing economies and rise in focus on end-to-end real-world evidence services are expected to provide lucrative Real World Evidence Solutions Market opportunity during the forecast period.
The global Real World Evidence Solutions Market size was valued at $1.4 billion in 2021, and is projected to reach $5 billion by 2031, growing at a CAGR of 13.7% from 2022 to 2031. The real-world evidence solutions market is segmented on the basis of component, application, end user and region. By component, it is segmented into datasets and consulting services. On the basis of application, it is segmented into market access & reimbursement/coverage decisions, drug development and approvals, post market surveillance, medical device development and approvals and others. By end user, it is segmented into pharmaceuticals and medical device companies, healthcare providers, healthcare payers and others. Region-wise, it is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
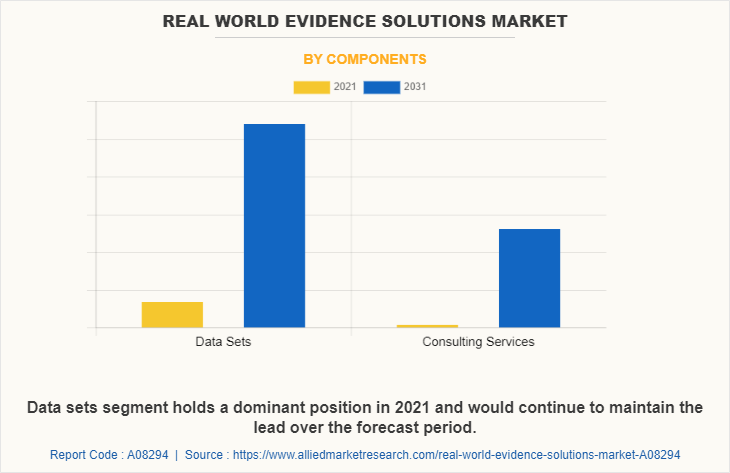
Component Segment review
By component, the RWE Solutions Market is segmented into datasets and consulting services. In 2021, the datasets segment accounted for the largest share of the market. The dominance of this segment can be attributed to rise in prevalence of chronic and autoimmune disorders and the increase in adoption of artificial intelligence-based RWE solutions by pharma companies.
The consulting services segment is anticipated to grow at a significant rate during the forecast period. Owing to increase in the demand for consulting services providers to provide services to ease the drug development process and accelerate the clinical trials process to get fast market approval.
Application Segment Review
By application, the RWE Solutions Industry is segmented into market access & reimbursement/coverage decisions, drug development and approvals, post-market surveillance, medical device development and approvals, and others. The market access & reimbursement/coverage decisions segment exhibited the highest Real World Evidence Solutions Market share in 2021, and is anticipated to lead during the forecast period.
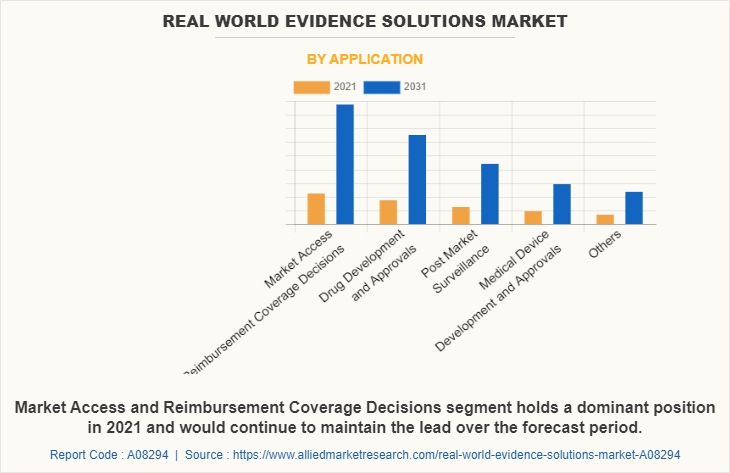
The drug development approvals segment is anticipated to grow at a significant rate during the forecast period owing to an increase in the prevalence of chronic diseases and rise in demand for novel drug development therapies and medical devices, which increase the demand for drug approvals process by using real-world evidence solutions.
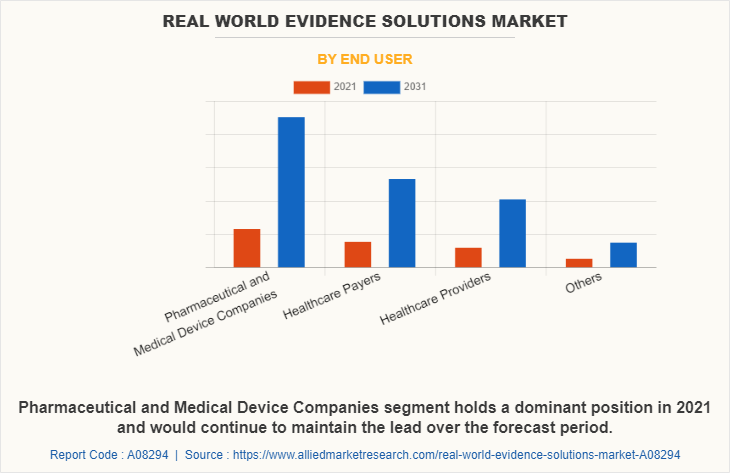
End User Segment Review
By end user, the pharmaceutical and medical device companies segment was the highest revenue contributor to the market owing to rise in prevalence of chronic diseases and an increase in R&D activities.
The healthcare payers are expected to witness considerable market growth during the forecast period, owing to increase in number of service providers and rise in the adoption of technologically advanced real-world evidence solutions.
Region Segment Review
Region wise, the real-world evidence solutions market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for a majority of the real-world evidence solutions market share in 2021 and is anticipated to remain dominant during the forecast period owing to increase in demand for real-world evidence-based solutions or services, the shift toward value-based care instead of volume and the strategies they adopt for their product development.
Asia-Pacific was the second largest contributor to the market in 2021, and is expected to register the fastest CAGR during the forecast period, owing to rise in geriatric population and increase in the prevalence of cancer and other chronic diseases across the region.
The key players that operate in the real-world evidence solutions market are Clarivate PLC, Clinigen Group PLC, Cognizant Technology Solution Corporation, Elevance Health Inc, Flatiron Health, IBM Corporation, ICON PLC, IQVIA, Medpace Holding Corporation, Oracle Corporation, Parexel International Corporation, Perkin Elmer Inc, SAS Institute Inc, Symphony Innovation LLC, and Syneos Health Inc.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the real world evidence solutions market analysis from 2021 to 2031 to identify the prevailing real world evidence solutions market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the real world evidence solutions market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global real world evidence solutions market trends, key players, market segments, application areas, and market growth strategies.
Real World Evidence Solutions Market Report Highlights
| Aspects | Details |
| By Components |
|
| By Application |
|
| By End User |
|
| By Region |
|
| Key Market Players | Oracle Corporation, ICON PLC, Syneos Health Inc, Clarivate plc, SAS Institute Inc, Cognizant Technology Solutions Corp., Perkin Elmer Inc, Medpace Holding Corporation, Symphony Innovation LLC, Elevance Health Inc, IBM Corporation, IQVIA, Clinigen Group plc, Flatiron Health, Parexel International Corporation |
Analyst Review
This section provides various opinions of top-level CXOs in the real-world evidence solutions market. In accordance with several interviews conducted, the real-world evidence solutions market is expected to witness significant growth in the future, owing to increase in demand for real-world evidence services for clinical research activities, drug development, increase in healthcare expenditure and high number of RWE service providers.
According to the perspectives of CXOs, the global real-world evidence solutions market is expected to witness a steady growth in the future. Rise in geriatric population and increase in number of chronic and various other diseases, the adoption of technologically advanced devices and services, growth in healthcare expenditure are the key factors drives the growth of the market. However, the reluctance to rely on real-world data or studies is expected to hamper the growth of the market up to some extent over the forecast period.
On the contrary, increasing in the number of RWE service providers and increase in need of end-to-end RWE services, are expected to provide opportunities for the growth of the global real-world evidence solutions market in future.
Further, North America is expected to witness highest growth, in terms of revenue, owing to the increase in demand for real-world evidence-based solutions or services, the shift toward value-based care instead of volume and rise in adoption of various strategies by key players for product development in the region are the factors that propel the growth of the market.
Asia-Pacific was the second largest contributor to the market in 2021, and is expected to register the fastest CAGR during the forecast period, owing to rise in prevalence of chronic diseases and increase in geriatric population and rise in number of market players that provide RWE services for the smooth conduct of drug development process or clinical trials.
The upcoming trends are are increase in prevalence of cancer and chronic diseases, which increase the demand for the development of novel drugs and medical devices across the world.
The forecast period in the report is from 2022 to 2031
North America is the largest regional market for Real World Evidence Solutions
The global real-world evidence solutions market was valued at $1,369.3 million in 2021
Clarivate PLC, Clinigen Group PLC, Cognizant Technology Solution Corporation, Elevance Health Inc, Flatiron Health, IBM Corporation are the top companies to hold the market share in Real World Evidence Solutions
o, there is no value chain analysis provided in Real World Evidence Solutions report.
2021 base year calculated in the Real World Evidence Solutions Market report
The global real-world evidence solutions market is projected to reach $4,993.24 million by 2031, registering a CAGR of 13.7% from 2022 to 2031
Loading Table Of Content...


