Hepatitis E Diagnostic Tests Market Research, 2031
The global hepatitis E diagnostic tests market size was valued at $61.7 million in 2021, and is projected to reach $93.5 million by 2031, growing at a CAGR of 4.2% from 2022 to 2031. Hepatitis E is a liver disease caused due to infection of hepatitis E virus (HEV). It includes inflammation to the liver along with some symptoms such as jaundice and nausea. The infection is usually transmitted through oral-fecal route, where drinking water contaminated with fecal residues remains the main cause of infection. In addition, vertical transmission that is from pregnant women to the baby, infected blood products used in medical treatments and intake of uncooked meat and meat products from hepatitis E infected animals are the major factors for the spread of infection.
Furthermore, hepatitis E presents is a hyper endemic disease in low-resource countries where there is poor sanitation which leads to fecal contamination of food and water supplies. Inappropriate sanitation in the underdeveloped and developing countries is also the main cause of this infection and these regions are the main targets for spread of diseases such as hepatitis E and others. This leads to rise in number of hepatitis infection cases, which further rises the demand for hepatitis E diagnostic test kits in this region.
Furthermore, hepatitis E can also lead to serious health threats to patients with pre-existing chronic liver disease and organ-transplant recipients on immunosuppressive therapy, which results in severe liver disease and death of the patient. In addition, during epidemics, fulminant hepatitis E is witnessed on a large scale among pregnant women, which is typically most severe during the third trimester of pregnancy. According to World Health Organization, 20–25% of pregnant women are at high risk and may die if infected by hepatitis E in third trimester. This highlights the need for early diagnosis of this infection which rises the demand for hepatitis E diagnostic tests kits, and propels the growth of the hepatitis E diagnostic tests market size.
In addition, rise in prevalence of hepatitis E, rise in awareness about hepatitis E and its diagnosis among people, and rise R&D activities in the diagnostic field are the driving factors for the hepatitis E diagnostic tests market share. These drivers provide a favorable environments for increase in the sales of the products and also for the growth of the overall market. However, the market may be somewhat constrained throughout the anticipated period due to complications in handling the kits and limitation in its storage conditions.
Impact of COVID-19 Hepatitis E Diagnostic Tests Market
Coronavirus Severe acute respiration syndrome (SARS-CoV-2) is an infectious disease caused by the novel coronavirus (COVID-19), which originated in the Wuhan district in China in the late 2019, and since has spread to 212 countries. WHO declared COVID-19 as a pandemic on March 11, 2020, and by September 21, 2022, over 613 million people have been infected globally with over 65.3 lakhs deaths. COVID-19 symptoms include fever, cough, and shortness of breath.
The COVID-19 outbreak is anticipated to have a negative impact on growth of the global hepatitis E diagnostic tests market. A survey was carried out by the World Hepatitis Alliance (WHA), a network of more than 300 community-based organizations across 100 countries in March–April, 2020. According to the survey, there was interruptions in the services, along with delays in implementing major programs regarding the hepatitis diagnosis and management. Moreover, the COVID-19 pandemic has affected the hepatitis prevention, testing, treatment, and vaccination services around the globe, which further lead to a negative impact on the hepatitis E diagnostic test market.
In addition, the COVID-19 have also imperiled the national elimination plan for viral hepatitis owing to diverted resources and attention of the healthcare sector. Furthermore, the government funding in hepatitis cases reduced due to COVID- 19 funding, which further reduced the patients opting for diagnosis and treatment of hepatitis E. In addition, decrease in the patient visit to the hospitals reduced during the pandemic, which further negatively affected the hepatitis E diagnostic tests market growth.
However, regularization of supply chain of medications and medical devices by key players and rise in the number of patient visits for investigation and treatment post pandemic is anticipated to fuel the growth of market after COVID-19 pandemic.
The hepatitis E diagnostic tests market is segmented on the basis of test type, by end user and region. On the basis of test type, it is divided into ELISA HEV IgM test, ELISA HEV IgG test, and RT-PCR test. On the basis of end user, the market is segmented into hospitals, diagnostic centers, and others.
On the basis of, the market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, UK, Italy, Spain, and rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, Saudi Arabia, South Africa, and rest of LAMEA).
Segment Review
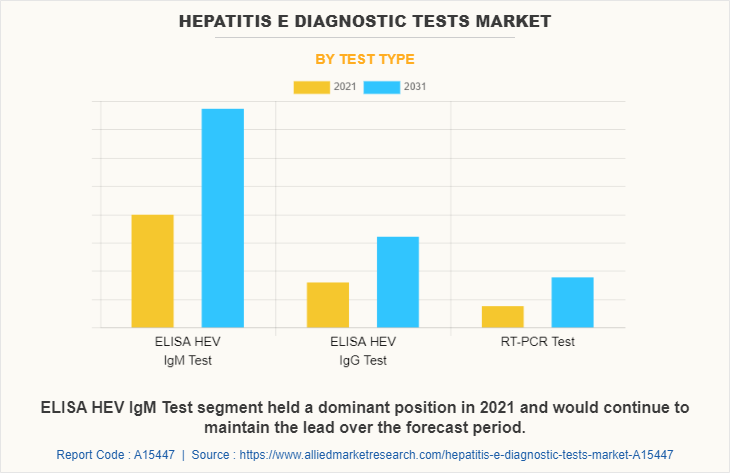
On the basis of test type, the HEV IgM ELISA Test segment registered the largest revenue for the year 2021, and is expected to grow at a highest CAGR during the hepatitis E diagnostic tests market forecast period owing to the rise in the investment by the key market players to manufacture the HEV IgM ELISA Test and others associated products to meet the rising demand of the healthcare sector.
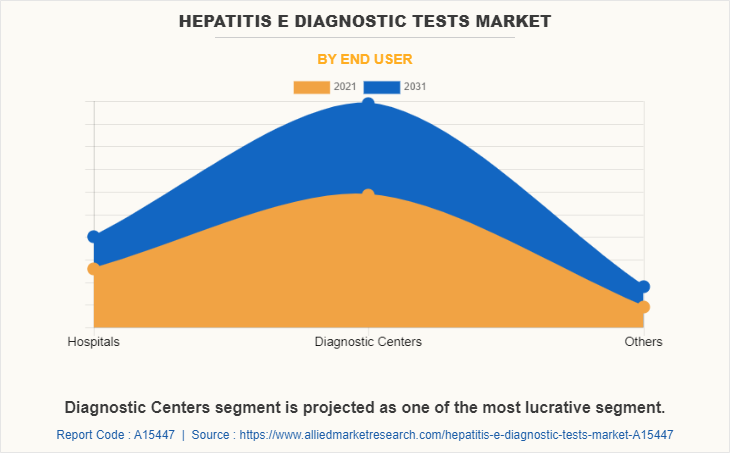
On the basis of end user, the diagnostic centers segment held the largest share of the hepatitis E diagnostic tests market in 2021 and is expected to grow at the highest CAGR during the forecast period, which is mainly rise in number of diagnostic centers around the globe due to the rise in demand for diagnostic services during the COVID-19 pandemic.
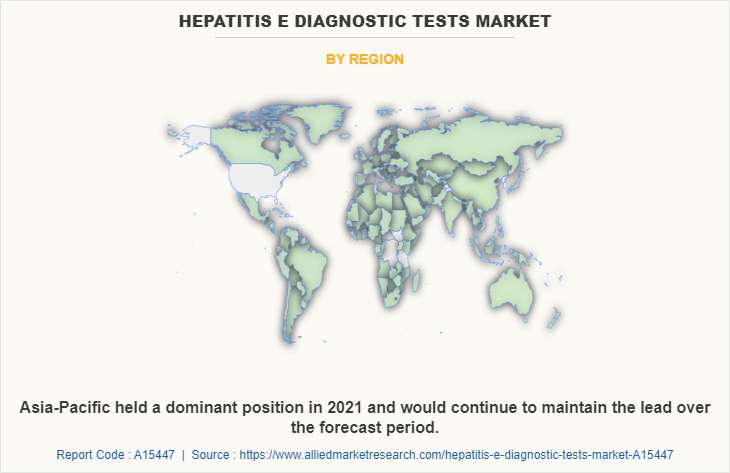
On the basis of region, Asia-Pacific acquired a major share in 2021, and is expected to witness the highest rate of CAGR for the hepatitis E diagnostic tests market during the forecast period owing to prevalence of various infectious diseases in this region. This, leads to rise in sale of diagnostic kits and increase the revenue share of this region.
Some of the major companies that operate in the global hepatitis E diagnostic tests industry are Altona Diagnostics GmbH, Beijing Wantai Biological Pharmacy Enterprise Co.,Ltd, Dia.Pro Diagnostic Bioprobes s.r.l, ELITechGroup, F. Hoffmann-La Roche Ltd., Fortress Diagnostics, Mikrogen Diagnostik, MP Biomedicals, PerkinElmer, Inc. (EUROIMMUN Medizinische Labordiagnostika AG) and Primerdesign Ltd.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the hepatitis e diagnostic tests market analysis from 2021 to 2031 to identify the prevailing hepatitis e diagnostic tests market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the hepatitis e diagnostic tests market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global hepatitis e diagnostic tests market trends, key players, market segments, application areas, and market growth strategies.
Hepatitis E Diagnostic Tests Market Report Highlights
| Aspects | Details |
| Market Size By 2031 | USD 93.5 million |
| Growth Rate | CAGR of 4.2% |
| Forecast period | 2021 - 2031 |
| Report Pages | 171 |
| By Test Type |
|
| By End User |
|
| By Region |
|
| Key Market Players | ELITechGroup, MP Biomedicals LLP, Primerdesign Ltd, Fortress Diagnostics, PerkinElmer, Inc, Altona Diagnostics, Beijing Wantai Biolog Pha Ent Co Ltd, Mikrogen GmbH, F. Hoffmann-La Roche Ltd., Dia.Pro - Diagnostic Bioprobes s.r.l |
Analyst Review
This section provides the opinions of the top-level CXOs operating in the global hepatitis E diagnostic tests market. According to the insights of the CXOs, the global hepatitis E diagnostic tests market is expected to exhibit high growth potential attributable to factor such as rise in in R&D activities in pharmaceutical industry for manufacturing of the diagnostic kits. In addition, increase in prevalence of hepatitis E cases and chronic hepatitis and surge in number advancements in the diagnosis and treatment are driving the market growth. Furthermore, the hepatitis E diagnostic tests market has gained interest of many pharmaceutical companies, owing to surge in demand for the diagnostic kits due to rise in the patient pool in the regions such as Asia-Pacific and LAMEA. Moreover, increase in number of geriatric patients, who are highly prone to viral and bacterial infections due to poor immunity, notably contributes toward the growth of the hepatitis E diagnostic tests market.
In addition, hepatitis E infection occurs around the world both as outbreaks and as sporadic cases. Outbreaks are mainly witnessed in countries with limited access to essential water, sanitation, hygiene and health services, which affects more than thousands of people in that region. However, government and non-government organizations here mainly focus on creating awareness about this condition and also provide funds for the prevention, diagnosis and treatment of hepatitis E infection.
The CXOs further added that Asia-Pacific is expected to witness the highest growth, in terms of revenue, owing to the presence of key players, rise in government initiatives, development in healthcare infrastructure, rise in prevalence of health issues, and is expected to register highest growth during the forecast period
Rise in prevalence of hepatitis E, rise in awareness about hepatitis E and its diagnosis among people, and rise R&D activities in the diagnostic field are the upcoming trends of Hepatitis E Diagnostic Tests Market in the world
Lever infection is the leading application of Hepatitis E Diagnostic Tests Market
Asia-Pacific is the largest regional market for Hepatitis E Diagnostic Tests.
Hepatitis E Diagnostic Tests is projected to reach $93.5 million by 2031, registering a CAGR of 4.2% from 2022 to 2031.
Some of the major companies that operate in the global Hepatitis E diagnostic tests market are Altona Diagnostics GmbH, Beijing Wantai Biological Pharmacy Enterprise Co.,Ltd, Dia.Pro Diagnostic Bioprobes s.r.l, ELITechGroup, F. Hoffmann-La Roche Ltd., Fortress Diagnostics, Mikrogen Diagnostik, MP Biomedicals, PerkinElmer, Inc. (EUROIMMUN Medizinische Labordiagnostika AG) and Primerdesign Ltd.
The base year is 2021 in Hepatitis E Diagnostic Tests market
Yes, the Hepatitis E Diagnostic Tests market report provides PORTER Analysis
Loading Table Of Content...



