Macular Degeneration Treatment Market Research, 2033
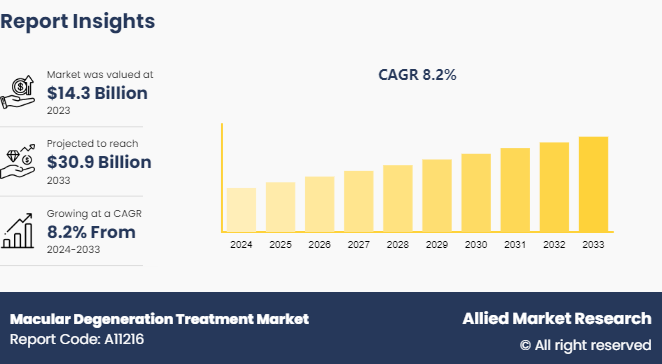
The global macular degeneration treatment market size was valued at $14.3 billion in 2023, and is projected to reach $30.9 billion by 2033, growing at a CAGR of 8.2% from 2024 to 2033.
Market Introduction
Macular degeneration, an eye condition affecting numerous people worldwide, demands effective treatment strategies. Currently, treatment options primarily aim to slow down disease progression and manage symptoms. One common approach involves injections of anti-VEGF drugs directly into the eye, targeting abnormal blood vessel growth. These injections often help to reduce swelling and preserve vision, particularly in the wet form of macular degeneration.
Another method involves photodynamic therapy, which employs laser treatment combined with a light-sensitive medication to selectively destroy abnormal blood vessels. Additionally, lifestyle changes such as adopting a nutrient-rich diet, quitting smoking, and wearing UV-protective sunglasses can aid in managing the condition. While these treatments offer significant advancements in combating macular degeneration, ongoing research attempts to discover more effective therapies to enhance vision preservation and improve patients' quality of life are gaining importance.
Key Takeaways
- The macular degeneration treatment market study covers 20 countries. The research includes a segment analysis of each country in terms of value ($billion) for the projected period from 2024 to 2033.
- More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major macular degeneration treatment industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrates high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach intends to provide a balanced view of global markets and to assist stakeholders in making informed decisions in order to achieve their most ambitious growth objectives.
Key Market Dynamics
The global prevalence of macular degeneration treatment industry is on the rise, propelled by sedentary lifestyles, obesity, and poor dietary choices. This surge in patient count intensifies the demand for effective treatment options. Furthermore, concerted efforts through awareness campaigns and educational programs emphasize the criticality of regular eye screenings, early detection, and the array of available treatment modalities. These initiatives play a major role in expanding the macular degeneration treatment market by empowering individuals to take proactive steps in preserving their vision. As people become more aware about the harmful consequences of this condition and the scope for intervention, there is a growing momentum towards addressing macular degeneration comprehensively. This collective push towards prevention, detection, and treatment signifies a crucial shift towards prioritizing ocular health on a global scale and thus supporting macular degeneration treatment market growth.
However, the high cost of treatment poses a significant obstacle to the advancement of macular degeneration treatment. Despite promising research and innovative treatments, the financial burden associated with these medical interventions threatens to hinder progress. Patients facing this extreme health condition often find themselves struggling with excessive expenses, limiting their access to the treatment. As the prevalence of macular degeneration continues to rise, addressing the affordability of care becomes increasingly urgent. Without concerted efforts to alleviate the financial strain on patients and healthcare systems, the growth and accessibility of effective treatments is hindered.
Analysis of Macular Degeneration Treatment Market
As the geriatric population continues to expand, so does the demand for effective treatments for age-related ailments such as macular degeneration. This degenerative eye condition, prevalent among older adults, significantly impacts vision, leading to blurred or distorted vision and, in severe cases, blindness. With a growing number of individuals entering their elderly years, the need for accessible and advanced treatments becomes important.
Macular degeneration not only affects individuals' quality of life but also poses significant challenge on healthcare systems worldwide for effective and economical treatment. Consequently, there is a pressing need for innovative approaches in treating this condition, ranging from pharmaceutical interventions to advancements in surgical techniques and assistive technologies. Meeting this demand will not only enhance the well-being of aging populations but also drive progress in the field of ophthalmology, offering hope for improved outcomes and quality of life for millions of individuals facing this extreme condition.
According to WHO, by 2030, the global population of individuals aged 60 and above is expected to reach one billion, constituting one in six people worldwide. By 2050, this demographic will double to 2.1 billion, with the number of those aged 80 or older tripling to 426 million. This demographic shift highlights a significant trend toward an aging population, posing challenges and opportunities for healthcare, social services, and economic systems worldwide. As longevity increases and birth rates decline, societies must adapt policies and infrastructure to accommodate the needs and contributions of older adults, ensuring their well-being and integration into society.
Market Segmentation
The macular degeneration treatment market share is segmented into type, stage of disease, route of administration, end user, and region. On the basis of type, the market is divided into dry age-related macular degeneration and wet age-related macular degeneration. As per stage of disease, the market is segregated into early stage, intermediate stage, and late stage. By route of administration the market is segmented on the basis of intravenous and intravitreal. On the basis of end user the market is diverged into hospitals & clinics, ambulatory surgical centers, and others. Region wise, the macular degeneration treatment market share is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional Market Outlook
The North America region has emerged as a dominant force in driving the demand for macular degeneration treatment growth. With an increasing prevalence of age-related macular degeneration (AMD), a condition affecting the central vision, the demand for effective treatments has surged. Macular degeneration treatment plays a major role in addressing this concern, offering effective solutions to alleviate symptoms and potentially halt disease progression. The region's robust healthcare infrastructure, coupled with advanced research and development efforts, has fueled the growth in demand. Additionally, a growing aging population and rising awareness about eye health have further propelled the need for innovative treatment options and thus providing macular degeneration treatment market opportunity.
In February 2023, Apellis Pharmaceuticals, Inc. announced the FDA approval for SYFOVRE (pegcetacoplan injection) in preventing geographic atrophy (GA) stemming from age-related macular degeneration (AMD) . As the first and sole FDA-endorsed therapy for GA, SYFOVRE offers hope to over one million Americans and five million individuals globally affected by this debilitating condition, a leading cause of blindness. This achievement marks a significant advancement in addressing the urgent medical needs of those grappling with AMD-related complications, positioning Apellis Pharmaceuticals at the forefront of biopharmaceutical innovation in the fight against vision impairment.
Competitive Landscape
The major players operating in the macular degeneration treatment market forecast include REGENXBIO Inc., Regeneron, Panoptica, Novartis AG, Bausch Health Companies Inc., Pfizer Inc., F. Hoffmann-La Roche AG, Astellas Pharma Inc., Thermo Fischer Scientific, Inc, and Aerie Pharmaceutical Inc. Other players in macular degeneration treatment market size analysis include Amgen Inc. and Santen Pharmaceutical Co., Ltd.
Recent Key Strategies and Developments
- In June 2022, Biogen Inc. and Samsung Bioepis Co., Ltd. introduced BYOOVIZ (ranibizumab-nuna) in the U.S., a biosimilar of LUCENTIS (ranibizumab) , approved by the FDA in September 2021 for neovascular age-related macular degeneration, macular edema following retinal vein occlusion, and myopic choroidal neovascularization. Priced at $1, 130 per vial for a 0.5 mg intravitreal injection, BYOOVIZ is 40% cheaper than LUCENTIS. With healthcare provider engagement underway, along with promotional efforts and collaborations with professional societies and patient advocacy groups, BYOOVIZ is now commercially available through major U.S. distributors.
- In December 2020, Janssen Pharmaceuticals, Inc., a subsidiary of Johnson & Johnson, acquired the rights to Hemera Biosciences, LLC's pioneering gene therapy, HMR59. This therapy, administered as a single outpatient intravitreal injection, targets geographic atrophy, a severe form of age-related macular degeneration (AMD) threatening vision. The acquisition highlights Janssen's commitment to advancing treatments for weakening eye conditions. This strategic move signals a significant step toward enhancing patient care and combating the impact of AMD on global health.
Industry Trends
- In August 2023, Astellas Pharma Inc. secured FDA approval for IZERVAY to address geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The product is positioned as a novel complement C5 inhibitor, IZERVAY is the only approved treatment demonstrating a statistically significant reduction (p<0.01) in GA progression rates at the 12-month primary endpoint across two Phase 3 clinical trials. This is a significant advancement in combating the weakening effects of AMD, offering hope to patients affected with vision loss due to this condition.
- In August 2023, Regeneron Pharmaceuticals, Inc. got the U.S. FDA approval for EYLEA HD (aflibercept) Injection 8 mg to address wet age-related macular degeneration (wAMD) , diabetic macular edema (DME) , and diabetic retinopathy (DR) . The recommended regimen entails an initial three-month period of monthly 8 mg injections, followed by injections every 8 to 16 weeks for wAMD and DME, and every 8 to 12 weeks for DR. This approval highlights EYLEA HD's efficacy in treating these sight-threatening conditions, offering patients a regimen that balances effectiveness with reduced frequency of injections, enhancing treatment adherence and outcomes.
Key Sources Referred
- Macular Degeneration Association (MDA)
- American Macular Degeneration Foundation (AMDF)
- International Agency for the Prevention of Blindness (IAPB)
- Association for Research in Vision and Ophthalmology (ARVO)
- Regeneron
- F. Hoffmann-La Roche AG
- Astellas Pharma Inc.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the macular degeneration treatment market analysis from 2024 to 2033 to identify the prevailing macular degeneration treatment market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the macular degeneration treatment market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global macular degeneration treatment market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global macular degeneration treatment market trends, key players, market segments, application areas, and market growth strategies.
Macular Degeneration Treatment Market Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 30.9 Billion |
| Growth Rate | CAGR of 8.2% |
| Forecast period | 2024 - 2033 |
| Report Pages | 295 |
| By Type |
|
| By Stage Of Disease |
|
| By Route Of Administration |
|
| By End User |
|
| By Region |
|
| Key Market Players | Astellas Pharma Inc., REGENXBIO Inc., Pfizer Inc., Panoptica, F. Hoffmann-La Roche AG, Regeneron, Thermo Fischer Scientific, Inc., Bausch Health Companies Inc, Novartis AG, Aerie Pharmaceutical Inc. |
| Other Players | Stryker Corporation |
As the geriatric population continues to expand, so does the demand for effective treatments for age-related ailments such as macular degeneration.
The hospitals & clinics sub-segment is expected to dominate throughout the forecast period.
North America region is anticipated to hold a dominant share in macular degeneration owing to its advanced healthcare infrastructure, robust research facilities, and increasing awareness campaigns.
The global macular degeneration treatment market was valued at $14.62 billion in 2023.
The major players operating in the macular degeneration treatment market include REGENXBIO Inc., Regeneron, Panoptica, Novartis AG, Bausch Health Companies Inc., Pfizer Inc., F. Hoffmann-La Roche AG, Astellas Pharma Inc., Thermo Fischer Scientific, Inc, and Aerie Pharmaceutical Inc.
Loading Table Of Content...



