Mitral Valve Disease Market Research, 2031
The global mitral valve disease market size was valued at $2.5 billion in 2021, and is projected to reach $5.7 billion by 2031, growing at a CAGR of 8.8% from 2022 to 2031. The mitral valve is a valve located in the heart that separates the left atrium from the left ventricle. It consists of two leaflets that open and close to allow blood to flow from the left atrium to the left ventricle during the heart's blood-pumping cycle. Mitral valve disease is a medical condition that affects the mitral valve, which is one of the four valves in the heart.
Some common types of mitral valve diseases include mitral valve regurgitation, mitral valve stenosis, and mitral valve prolapse. Mitral valve disease can cause a range of symptoms, including fatigue, shortness of breath, chest pain, palpitations, and swelling in the legs or abdomen. The severity of the symptoms depends on the extent of the valve damage and the underlying cause of the disease. Treatment options for mitral valve disease may include medications, surgery, or minimally invasive procedures to repair or replace the affected valve or cardiac resynchronization therapy in some cases.
Market dynamics
Growth of mitral valve disease market size is mainly due to the rise in incidences of mitral valve diseases, surge in demand for advance mitral valve treatment medications and devices, and rise in awareness among people regarding mitral valve disease and its advanced treatment options. This can be attributed to several factors such as an aging population, increasing prevalence of risk factors such as high blood pressure, high cholesterol, and changes in lifestyle habits. This is increasing the demand for mitral valve treatment products and further driving the growth of the market.
In addition, advancements in medical technology and improved treatment techniques have led to better management of mitral valve diseases, which may contribute to the growth of the market. For instance, MitraClip is a specific type of TMVR device that is used to repair the mitral valve. The device consists of a small clip that is attached to the valve leaflets to help them close properly. MitraClip has been shown to be effective in reducing mitral regurgitation and improving symptoms in patients who are not candidates for traditional surgery. Such technological advancements lead to the rise in adoption of these advanced devices and thus contribute to the mitral valve disease market growth.
Furthermore, surge in demand for minimally invasive procedures for treatment of mitral valve diseases and subsequent development of minimally invasive products fosters the market growth. For instance, in September 2019, Medtronic Plc has received the U.S. Food and Drug Administration (FDA) approval to conduct an early feasibility study (EFS) of its Intrepid transcatheter mitral valve replacement (TMVR) system. This system is designed to replace the mitral valve in the heart using a minimally invasive transfemoral access approach. Such new developments in the sector is boosting the growth during mitral valve disease market forecast.
On the other hand, factor such as stringent regulations by the governing bodies for the development, usage and approval of mitral heart valves and medications acts as a restraint to the growth of market. In addition, manufacturing of mitral valve repair, replacement and cardiac resynchronization devices must comply with strict standards to ensure its safety and quality. Such regulations regarding development and usage of mitral valve, hinder the market growth. In addition, high cost of mitral valve repair and replacement surgeries and risk associated with the procedure also caused hindrance to the market growth.
The COVID-19 outbreak has had a negative impact on the growth of the mitral valve disease industry. The pandemic has caused partial or complete shutdown of production facilities in many countries, leading to a suspension of production activities in most industrial units across the world. This has affected the manufacturing and development activities related to mitral valve treatment therapeutics and devices.
The prolonged lockdowns in major countries such as the U.S., China, Japan, India, and Germany have also resulted in a decrease in patient visits to hospitals and clinics, which has affected the demand for mitral valve treatment procedures. Additionally, healthcare systems have been overwhelmed with COVID-19 patients, resulting in a delay of procedures such as mitral valve treatment surgeries.
Overall, the COVID-19 pandemic has had a significant impact on the mitral valve disease industry, leading to a decrease in the number of surgeries performed and a decrease in the demand for mitral valve repair and replacement procedures. However, after the pandemic, regularization of supply chain of medications and medical devices by key players is anticipated to expand the growth of the market. Furthermore, resumption of surgeries post pandemic will increase the demand for mitral valve repair, replacement and cardiac synchronization therapies and hence will augment the market growth.
Segmental Overview
The mitral valve disease market is segmented on the basis of treatment type, indication, end user and region. By treatment type, the market is categorized into mitral valve repair, mitral valve replacement, cardiac resynchronization therapy (CRT) and mitral valve therapeutics. The mitral valve replacement segment is categorized into mechanical valves and biological valves. On the other hand, the mitral valve therapeutics segment is divided into beta blockers, diuretics, anticoagulants and others. By indication, the market is bifurcated into mitral valve stenosis, mitral valve prolapse and mitral valve regurgitation. Further, mitral valve regurgitation segment is divided into primary (degenerative) mitral valve regurgitation and secondary (functional) mitral valve regurgitation.
By end user, the market is segregated into hospitals, ambulatory surgical centers and others. Region wise, the market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, the UK, Italy, Spain, and the rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and rest of LAMEA).
By Treatment Type:
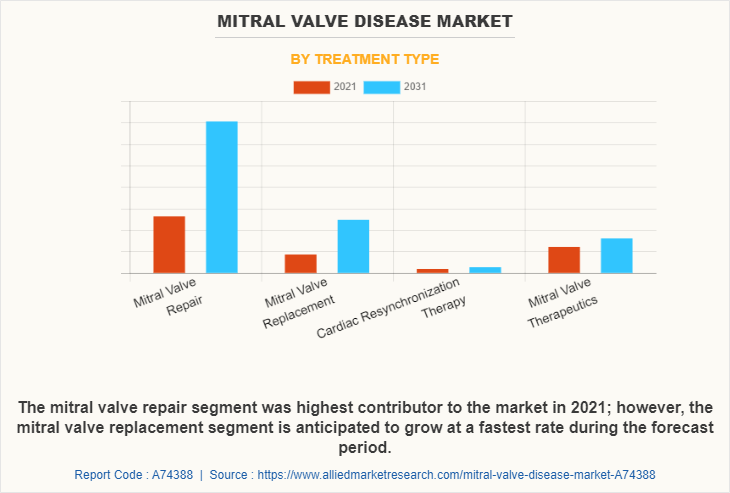
The mitral valve repair segment accounted for a major mitral valve disease market share in terms of revenue in 2021. This growth is attributed to the advantages offered by the repair procedure such as preservation of the natural valve, and faster recovery. Mitral valve repair allows the patient to keep their native heart valve, as it functions better, and does not require any lifelong anticoagulants. As it is a less invasive procedure than replacement, it has a faster recovery and the patient experiences less pain.
On the other hand, the mitral valve replacement segment is anticipated to grow at the fastest rate during the forecast period. Replacement of the mitral valve may be a better option in certain situations, when the valve is severely damaged or if there is a high risk of recurrent problems after the repair. In addition, mitral valve replacement can provide a durable, long-lasting solution for patients with mitral valve disease, and thus have a rising demand in the market.
By Indication:
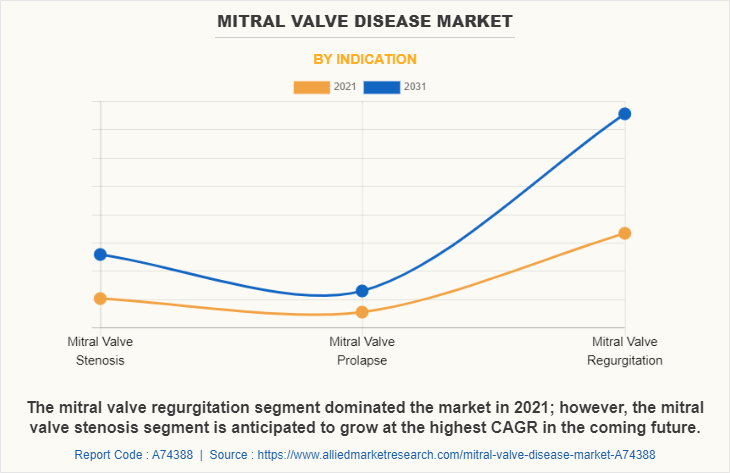
The mitral valve regurgitation segment was the largest segment in terms of revenue in 2021, and mitral valve stenosis segment is anticipated to grow at a fastest rate during the forecast period. Mitral valve regurgitation segments growth is attributed to the development of advanced mitral valve devices by the key players for treatment of mitral regurgitation along with rise in incidences of mitral prolapse leading to mitral regurgitation.
On the other hand, the growth of mitral valve stenosis segment during the forecast period is anticipated to be driven by high prevalence of mitral stenosis around the globe especially in developing region and development of annuloplasty and balloon valvuloplasty for stenosis treatment.
By End User:
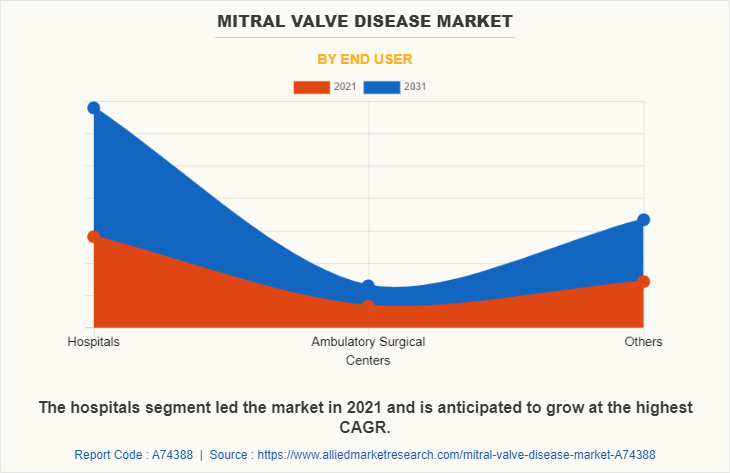
The hospitals segment held the largest market share in 2021, and is expected to grow at a fastest rate during the forecast period, as hospitals are the primary setting in which mitral valve replacement and repair procedures are performed. In addition, hospitals that specialize in heart surgery have highly trained staff which can reduce the risk of complications during and after surgery. Thus contributing to the hospital segment growth.
By Region:
The mitral valve disease market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for a major mitral valve disease market share in terms of revenue in 2021 and is expected to maintain its dominance during the forecast period. This is primarily attributed to its well-developed healthcare infrastructure, higher awareness among consumers regarding mitral valve diseases, and the rise in the incidence of mitral valve diseases in this region. In addition, presence of major players in U.S. and its wide offering in mitral valve treatment sector is increasing the adoption of mitral valve products in North American population.
Asia-Pacific expected to grow at the highest rate during the forecast period. This is attributed to huge population base as well as growth in the purchasing power of populated countries, such as China and India. These countries offer lucrative opportunities for players operating in the mitral valve disease market. The improving healthcare infrastructure, rise in number of healthcare reforms, and increase in healthcare expenditures in the emerging markets are set to boost the demand for mitral valve repair, replacement and cardiac resynchronization devices. In addition, growing geriatric population and untapped market opportunities are contributing to the market growth.
COMPETITION ANALYSIS
Competitive analysis and profiles of the major players in the mitral valve treatment such as Valcare Medical, Affluent Medical, Abbott Laboratories, Corcym UK Limited, Edwards Lifesciences Corporation, Medtronic Plc., ShockWave Medical, Inc., Artivion, Inc., Labcor Laboratorios Ltda, Braile Biomedica, Zydus Lifesciences Limited, Teva Pharmaceutical Industries Ltd., Pfizer Inc., Bayer AG, and Novartis AG are provided in this report. Major players have adopted divestment, agreement, product approval, investment, clinical trials, patent, branding, acquisition, partnership, new product development and others, as the key developmental strategies to improve the product portfolio of the mitral valve treatment medications and devices.
Recent Approvals in Mitral Valve Disease Market
- In January 2020, Abbott received the CE Mark approval of Abbott's Tendyne Transcatheter Mitral Valve Implantation (TMVI) system used in the treatment of mitral regurgitation (MR) in patients who require a heart valve replacement and is now approved for use in Europe.
- In September 2021, Abbott received the U.S. Food and Drug Administration (FDA) approval for Epic Plus and Epic Plus Supra Stented Tissue Valves. These new devices are based on Abbott's Epic surgical valve platform, which improve therapy options for people with aortic or mitral valve disease.
Divestment in Mitral Valve Disease Market
- In June 2021, Corcym announced the launch of its operations globally, which is formed from the acquisition of the LivaNova Plc. heart valve business by Gyrus Capital. The new, independent, medical device company will be dedicated towards providing patients and heart surgeons with the best solutions to fight structural heart disease.
Investment in the Mitral Valve Disease Market
- In March 2019, Edwards Lifesciences Corporation has announced two strategic transactions involving companies with structural heart disease technologies. The transaction involves a $35 million investment in an exclusive right to acquire Corvia Medical, Inc. and the acquisition of certain assets of Mitralign, Inc, a company that specializes in developing devices for the treatment of mitral valve regurgitation.
New Product Development in Mitral Valve Disease Market
- In November 2021, Medtronic plc. presented early data for Intrepid transcatheter mitral valve replacement (TMVR) system in patients with severe, symptomatic mitral valve regurgitation (MR) utilizing the transfemoral access route. The data from first 5 patients enrolled in an Early Feasibility Study of the Intrepid Transfemoral System showed 100% survival and no stroke and none or trace MR in all implanted patients at 30 days.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the mitral valve disease market analysis from 2021 to 2031 to identify the prevailing mitral valve disease market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the mitral valve disease market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global mitral valve disease market trends, key players, market segments, application areas, and market growth strategies.
Mitral Valve Disease Market Report Highlights
| Aspects | Details |
| Market Size By 2031 | USD 5.7 billion |
| Growth Rate | CAGR of 8.8% |
| Forecast period | 2021 - 2031 |
| Report Pages | 374 |
| By Treatment Type |
|
| By Indication |
|
| By End User |
|
| By Region |
|
| Key Market Players | Valcare Medical, Abbott Laboratories, ShockWave Medical, Inc., Teva Pharmaceutical Industries Ltd., Corcym UK Limited, Bayer AG, Braile Biomedica, Edwards Lifesciences Corporation, Affluent Medical, Novartis AG, Medtronic plc, Pfizer Inc., Artivion, Inc., Zydus Lifesciences Limited, Labcor Laboratorios Ltda |
Analyst Review
The demands for efficient and high-quality heart valve devices for the treatment of mitral valve diseases is significantly high, which is expected to offer profitable opportunities for the expansion of the market. Moreover, rising awareness among the people regarding mitral valve repair and replacement procedures is anticipated to boost the market growth.
The increase in incidence of mitral valve diseases such as mitral stenosis and mitral regurgitation in the population of developed as well as developing regions has largely contributed toward the revenue growth in 2021 and is expected to maintain this trend throughout the forecast period. Moreover, market players are investing in the development of technologically advanced mitral valves with increased clinical indications and improved efficacy and quality, which is expected to drive the growth of the market.
In addition, there has been an increasing trend toward minimally invasive mitral valve repair or replacement procedures, which can offer benefits such as reduced recovery time and fewer complications compared to traditional open-heart surgery. Transcatheter mitral valve repair or replacement (TMVR) is one example of a minimally invasive procedure that is gaining traction in the market. Moreover, the mitral valve disease market is becoming increasingly competitive, with a growing number of companies developing new treatments and technologies. This competition is driving innovation and leading to the development of new and more effective treatments for the disease, which is anticipated to augment the market growth.
Mitral valve stenosis, mitral valve prolapse and mitral valve regurgitation are the indications that causes mitral valve diseases.
Key factors driving the growth of the mitral valve disease market includes rise in incidence of mitral valve diseases, increase in demand for minimally invasive procedures, rise in geriatric population and increase in product approvals for mitral valve diseases treatment.
The mitral valve repair segment is the most influencing segment in the mitral valve disease market which is attributed to the advantages offered by the repair procedure such as preservation of the natural valve, and faster recovery. Mitral valve repair allows the patient to keep their native heart valve, as it functions better, and does not require any lifelong anticoagulants.
Top companies such as Valcare Medical, Affluent Medical, Abbott Laboratories, Corcym UK Limited, Edwards Lifesciences Corporation, Medtronic Plc., ShockWave Medical, Inc., Artivion, Inc., Labcor Laboratorios Ltda and Braile Biomédica hold a prominent position in the market.
The base year is 2021 in mitral valve disease market.
The forecast period for mitral valve disease market is 2022 to 2031
The market value of mitral valve disease market in 2031 is $5692.53 million.
The total market value of mitral valve disease market is $2447.83 million in 2021.
Loading Table Of Content...
Loading Research Methodology...


