Newborn Screening Instruments Market Research, 2033
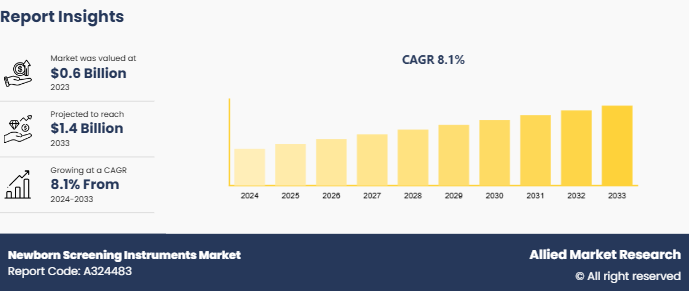
The global newborn screening instruments market size was valued at $0.6 billion in 2023, and is projected to reach $1.4 billion by 2033, growing at a CAGR of 8.1% from 2024 to 2033. The technological advancements in the screening devices such as improved accuracy, and integration with electronic health records, enhance the efficiency and reliability of the instruments and thereby drive the newborn screening instruments market growth.
Market Introduction and Definition
Newborn screening instruments are specialized medical devices designed to detect congenital disorders, metabolic diseases, and genetic conditions in newborns shortly after birth. These instruments play a crucial role in early diagnosis, enabling timely interventions that can prevent severe health complications, disabilities, or death. Key equipment includes mass spectrometers, pulse oximeters, hearing screening devices, and enzymatic assay systems, among others. These tools analyze blood samples, oxygen levels, or auditory responses to identify potential anomalies. Newborn screening instruments are essential components of public health programs, often supported by government mandates, as they enhance healthcare outcomes by facilitating early detection and treatment of conditions like phenylketonuria (PKU) , hypothyroidism, and cystic fibrosis.
Key Takeaways
- The newborn screening instruments market share study covers 20 countries. The research includes a segment analysis of each country in terms of value ($Billion) for the projected newborn screening instruments market forecast period 2024-2033.
- More than 1, 500 product type literatures, industry releases, annual reports, and other such documents of major newborn screening instruments industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrated high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and to assist stakeholders in making educated decisions in order to achieve their most ambitious growth objectives.
Key Market Dynamics
The newborn screening instruments market growth is shaped by several key drivers and restraints. A significant factor driving market growth is the rising incidence of congenital disorders. Newborns are increasingly being diagnosed with conditions such as metabolic disorders, hearing impairment, and congenital hypothyroidism, which require early detection for effective management. Timely screening helps prevent long-term complications and improve quality of life, fueling demand for advanced screening instruments. The growing awareness among parents and healthcare professionals about the importance of early diagnosis further supports the expansion of the market.
On the other hand, high initial costs associated with newborn screening instruments act as a restraint to market growth. Advanced technologies, such as tandem mass spectrometry and genetic testing platforms, require significant investment in equipment, installation, and maintenance. Additionally, the operational costs associated with training healthcare personnel and maintaining the required infrastructure can limit the adoption of these technologies, particularly in low-resource settings and developing countries.
Government initiatives and mandates play a crucial role in shaping the newborn screening instruments market. Many countries have implemented mandatory screening programs, supported by funding and policy frameworks. These initiatives ensure widespread adoption of screening practices, making early detection accessible to a larger population. Furthermore, continuous advancements in technology, such as improved accuracy, faster turnaround times, and integration with electronic health records, enhance the efficiency and reliability of newborn screening instruments. These innovations make screening more accessible and user-friendly, increasing the willingness of healthcare providers to adopt advanced systems.
Despite the challenges posed by high costs, the combination of rising congenital disorder rates, supportive government policies, and technological advancements creates significant growth opportunities for the newborn screening instruments market. However, addressing the cost barrier remains essential to ensure the widespread adoption of these life-saving technologies.
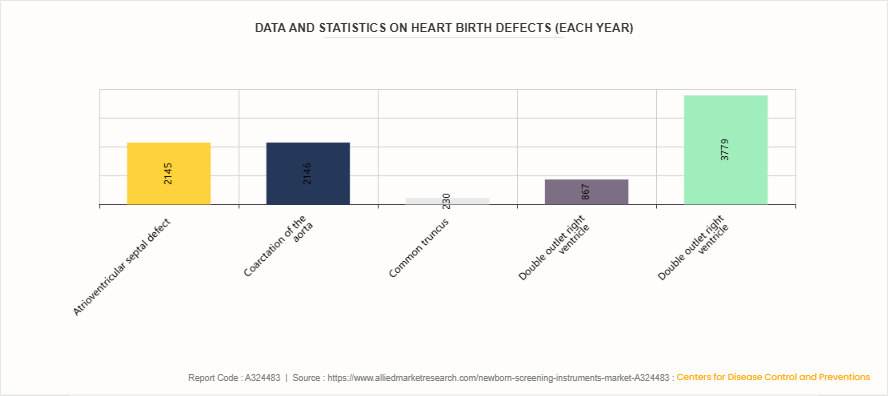
Data and Statistics on Heart Birth Defects (Each year)
According to the Centers for Disease Control and Prevention (CDC) data on heart birth defects, certain congenital heart defects affect thousands of newborns annually. The Atrioventricular septal defect and Coarctation of the aorta are the most common, affecting 2, 145 and 2, 146 babies, respectively. Other conditions such as Common truncus, Double outlet right ventricle, and Pulmonary valve atresia and stenosis impact 230, 867, and 3, 779 babies each year, respectively. These statistics underscore the importance of newborn screening instruments for early diagnosis and timely intervention, which are crucial in identifying congenital heart defects and ensuring better health outcomes for affected infants.
Market Segmentation
The newborn screening instruments industry is segmented into product type, application, end user, and region. On the basis of the product type, the market is categorized into mass spectrometers, pulse oximeters, hearing screening devices, and enzyme analyzers. As per application, the market is segmented into tandem mass spectrometry, DNA-based assays, immunoassays, and others. On the basis of end user, the market is divided into hospitals, diagnostic centers, and others. Region wise, it is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional/Country Market Outlook
The regional and country outlook for the Newborn Screening Instruments Market share highlights significant growth driven by advancements in healthcare infrastructure and government initiatives. Developed regions like North America and Europe lead the market, supported by well-established newborn screening programs, technological innovation, and rising awareness. In contrast, emerging economies in Asia-Pacific and Latin America are witnessing rapid growth due to increased healthcare funding and improved access to screening services. Countries such as China, India, and Brazil present lucrative opportunities, driven by large birth rates and expanding neonatal care facilities. However, disparities in healthcare access and program implementation remain challenges in certain regions.
Industry Trends
- In June 2022, Rady Children’s Institute for Genomic Medicine (RCIGM®) , announced a novel program to advance and evaluate scalability of a diagnostic and precision medicine guidance tool called BeginNGS, to screen newborns for approximately 400 genetic diseases that have known treatment options using rapid Whole Genome Sequencing (rWGS) . BeginNGS, developed through a research collaboration with Alexion, AstraZeneca’s Rare Disease group; Fabric Genomics; Genomenon; Illumina, Inc.; and TileDB, uses rWGS to diagnose and identify treatment options for genetic conditions before symptoms begin, an advancement over current pediatric uses of rWGS that focus mainly on children who are already critically ill.
Competitive Landscape
The major players operating in the Newborn Screening Instruments Market Size include Natus Medical Incorporated, Trivitron Healthcare, Medtronic Inc., Bio-Rad Laboratories Inc., PerkinElmer, GE Healthcare, Masimo Corporation, Waters Corporation, Thermo Fisher Scientific, and Hill-Rom Holdings Inc.
Recent Key Strategies and Developments
- In August 2023, Masimo, a global leader in noninvasive monitoring technologies, announced the full U.S. market release of the Stork smart home baby monitoring system. With the launch of Stork, parents for the first time can have access to a technology originally developed for the most challenging patients in hospitals, with a user interface that is a simple and easy-to-use app on a phone.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the Newborn Screening Instruments Market Analysis from 2024 to 2033 to identify the prevailing newborn screening instruments market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the newborn screening instruments market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global newborn screening instruments market trends, key players, market segments, application areas, and market growth strategies.
Newborn Screening Instruments Market , by Product Type Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 1.4 Billion |
| Growth Rate | CAGR of 8.1% |
| Forecast period | 2024 - 2033 |
| Report Pages | 163 |
| By Product Type |
|
| By Application |
|
| By End User |
|
| By Region |
|
| Key Market Players | Bio-Rad Laboratories Inc., Medtronic Inc., Thermo Fisher Scientific Inc, PerkinElmer, Natus Medical Incorporated., Hill-Rom Holdings Inc., Waters Corporation, GE Healthcare, trivitron healthcare pvt. ltd., Masimo Corporation |
The total market value of Newborn Screening Instruments Market was $ 0.6 billion in 2023.
The market value of Newborn Screening Instruments Market is projected to reach $ 1.4 billion by 2033.
The forecast period for Newborn Screening Instruments Market is 2024 to 2033
The base year is 2023 in Newborn Screening Instruments Market.
Newborn screening instruments are specialized medical devices designed to detect congenital disorders, metabolic diseases, and genetic conditions in newborns shortly after birth. These instruments play a crucial role in early diagnosis, enabling timely interventions that can prevent severe health complications, disabilities, or death.
Loading Table Of Content...


