Primary Biliary Cholangitis Therapeutics Market Research, 2032
The global primary biliary cholangitis therapeutics market size was valued at $684 million in 2022, and is projected to reach $1.4 billion by 2032, growing at a CAGR of 7.1% from 2023 to 2032. Primary biliary cholangitis (PBC), formerly known as primary biliary cirrhosis, is a chronic autoimmune liver disease that primarily affects the small bile ducts within the liver. It is characterized by the progressive destruction of these bile ducts, leading to impaired bile flow and subsequent liver damage. The exact cause of PBC is still unknown. However, it is believed to result from a combination of genetic, environmental, and immune system factors. Certain genetic predispositions and environmental triggers, such as infections or exposure to toxic substances, may contribute to the development of the disease. The ursodeoxycholic acid, obeticholic acid are the U.S. Food and Drug Administration (FDA) approved drugs for the treatment of primary biliary cholangitis.
Market Dynamics
The growth of the primary biliary cholangitis therapeutics market share is driven by the rise in cases of primary biliary cholangitis in Asia-Pacific. For instance, according to the review article “Current understanding of primary biliary cholangitis”, published in January 2021, the point prevalence of PBC was 33.8 per 100,000 in the Japanese population. As per the Research Outreach article, in May 2020, over 37,000 people in Japan are estimated to have primary biliary cholangitis (PBC). Rise in the cases of primary biliary cholangitis has resulted in increased demand for primary biliary cholangitis therapeutics.
Furthermore, the pharmaceutical industry continues to invest in research and development efforts to better understand the underlying mechanisms of PBC and develop innovative therapies. This includes exploring new drug targets, conducting clinical trials, and investigating combination therapies. Therefore, the rise in research and development regarding primary biliary cholangitis therapeutics drives the growth of the primary biliary cholangitis therapeutics market size during the forecast period. in June 2021, HighTide Therapeutics (“HighTide”), a clinical-stage biopharmaceutical company focused on the development of innovative therapies for non-viral chronic liver diseases, gastrointestinal diseases, and metabolic disorders, announced that the first patient has been dosed in Phase 2 PRONTO-PBC study of HighTide evaluating first-in-class HTD1801 in adult patients with primary biliary cholangitis (PBC).
Moreover, PBC is a chronic condition that requires long-term management. Despite the availability of certain therapies, there is still an unmet need for more effective treatments, particularly for patients who do not respond adequately to existing therapies. This drives the demand for innovative and targeted therapeutics to address the unmet medical needs in PBC. Hence, such factors drive the growth of the primary biliary cholangitis therapeutics market share.
However, lack of awareness regarding primary biliary cholangitis (PBC), early diagnosis of PBC, and limited treatment options are expected to hinder the growth of primary biliary cholangitis therapeutics market. On the contrary, an increase in the adoption of key strategies such as acquisition, product approval, and agreement by key players and advancements in primary biliary cholangitis therapeutics are expected to open new avenues for the growth of the primary biliary cholangitis therapeutics industry across the world during the forecast period.
The COVID-19 pandemic affected the primary biliary cholangitis therapeutics industry in a negative way, such as various healthcare industries were affected due to postponed of non-emergency services. The pandemic has disrupted research activities, including clinical trials, restrictions on patient recruitment and new product development. However, with relaxation in lockdowns and decline in COVID-19 cases, companies have re-started their processes to meet the demand for primary biliary cholangitis therapeutics. In addition, rise in product approvals, and increase in research and development of advanced medicine of primary biliary cholangitis therapeutics drive the growth of primary biliary cholangitis therapeutics market.
In addition, rise in awareness in pharmaceutical companies, and governments regarding supply chain hinderance of pharmaceutical products drives the growth of primary biliary cholangitis therapeutics market. For instance, government agencies in the U.S. and around the globe learned some tough lessons about the pharmaceutical supply chain after the COVID-19 pandemic landed, causing problems and highlighting weaknesses in key areas. In the session of "U.S. Government Investments in Pharmaceutical Manufacturing and Supply Chain and Future Pandemics for today" at the 2022 BIO International Conference, representatives from the U.S. health agencies shared lessons learned from COVID-19, and how government and industry have collaborated to avoid future supply chain crises. Moreover, the U.S. leaders stated that the government has learned since the beginning of the pandemic, steps taken since arrival of COVID-19, and plans the U.S. has put in place to avoid problems in the future. Thus, such trends are expected to bring stabilization in the market, and subsequently witness pre-pandemic growth rate during the forecast period.
Segmental Overview
The primary biliary cholangitis therapeutics market is segmented on the basis of drug type, distribution channel, and region. On the basis of drug type, the market is bifurcated into primary treatment and secondary treatment. The primary treatment segment is further categorized into obeticholic acid, ursodeoxycholic acid, and others (PPARα/PPARδ agonist, and ileal bile acid transport inhibitors). On the basis of distribution channel, it is segregated into hospital pharmacies, drugs stores and retail pharmacies, and online pharmacies. On the basis of region, the market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, the UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, Australia, India, South Korea, and Rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and Rest of LAMEA).
By Drug Type
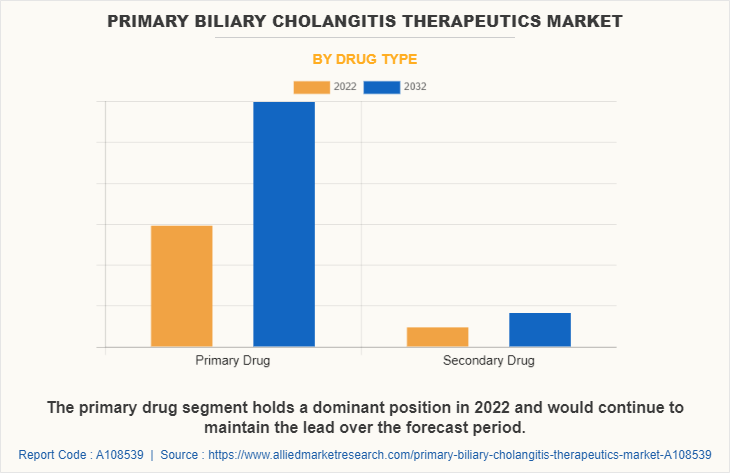
The primary biliary cholangitis therapeutics market is segmented into primary drug type and secondary drug type. The primary drug segment generated maximum revenue in 2022, owing to high adoption of primary drug for the treatment of PBC and the effectiveness of primary drug against PBC. The same segment is expected to witness the highest CAGR during the forecast period, owing to rise in patient suffering from PBC and availability of various primary drugs in pipeline.
By Distribution Channel
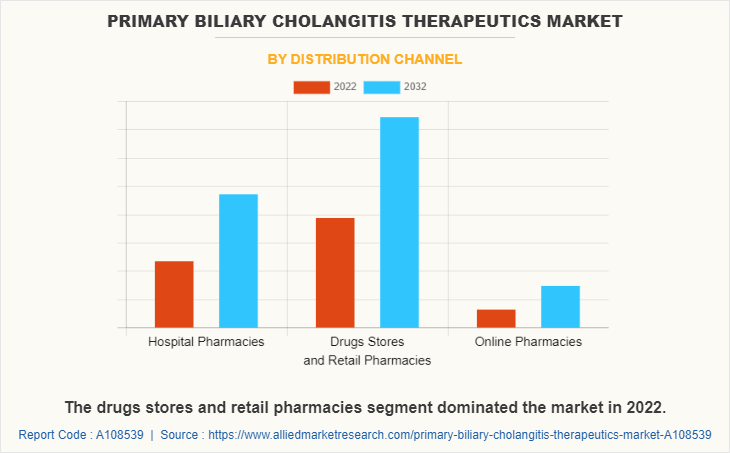
The primary biliary cholangitis therapeutics market is segregated into hospital pharmacies, drugs stores and retail pharmacies, and online pharmacies. The drugs stores and retail pharmacies segment dominated the market in 2022, owing to high sales of drugs from drugs stores and retail pharmacies. In addition, the geriatric population is more vulnerable to acquire a number of diseases, which increases dependence on drug stores, thus propel growth of the drugs stores and retail pharmacies segment. The online pharmacies segment is expected to witness the highest CAGR during the forecast period, owing to convenience in shopping, increase in e-commerce sales, improvements in logistics services, and ease in payment options. In addition, the easy accessibility and heavy discounts & offer provided by these online platforms drives the growth of online pharmacies during forecast period.
By Region
North America accounted for a major share of the primary biliary cholangitis therapeutics market in 2022 and is expected to maintain its dominance during the forecast period. The presence of several major players, such as CymaBay Therapeutics, Intercept Pharmaceuticals, Inc., COUR Pharmaceuticals and others; rise in number of clinical trials; availability of advanced healthcare facilities; and high healthcare expenditure from the government organizations in the region drive the growth of the market.
Furthermore, the existence of a sophisticated reimbursement structure that aims to reduce expenditure levels fosters the growth of the market. In addition, the U.S. is anticipated to contribute to a major share of the regional market and is expected to drive the growth of the primary biliary cholangitis therapeutics market throughout the forecast period. The presence of key players, high purchasing power, rise in research and development activities regarding primary biliary cholangitis therapeutics, and significant increase in capital income in developed countries. For instance, CymaBay Therapeutics, a U.S. based clinical-stage biopharmaceutical company focused on improving the lives of people with primary biliary cholangitis, engaged in developing Seladelpar (a first-in-class oral, selective, PPARδ agonist). Seladelpar has been shown to regulate critical metabolic and liver disease pathways in indications with high unmet medical need.
Asia-Pacific is expected to grow at the highest rate during the primary biliary cholangitis therapeutics market forecast period. The primary biliary cholangitis therapeutics market growth in this region is attributable to a rise in cases of primary biliary cholangitis as well as increase in purchasing power of populated countries, such as China and India. The countries in Asia-Pacific possess a huge population base, with China being the first having 1,411,778,724 population in 2020 and India is the second most populated country having 1,380,004,385 population in 2020. A rise in population along with longer life expectancy is thus expected to drive the growth of the market in Asia-Pacific during the forecast period.
COMPETITION ANALYSIS
Competitive analysis and profiles of the major players in the primary biliary cholangitis therapeutics market, such as ABC Farmaceutici S.p.a., Alkem Laboratories Limited, AMAGEN INDIA LIFE SCIENCES, GENFIT, Intercept Pharmaceuticals, Inc., Ipsen Pharma, Leeford Healthcare Ltd, Lupin, Sun Pharmaceutical Industries Ltd., and Zydus Lifesciences Limited. are provided in the report. There are some important players in the market such as ABC Farmaceutici S.p.a., Intercept Pharmaceuticals, Inc., GENFIT, Ipsen Pharma, Zydus Lifesciences Limited, and Lupin. These players have adopted agreement, acquisition, product approval, clinical trials, and partnership as their key developmental strategies to improve their product portfolio.
Recent Acquisition in the Primary Biliary Cholangitis Therapeutics Market
In March 2023, Ipsen announced that it has completed the acquisition of Albireo Pharma, Inc., a leading innovator in bile-acid modulators to treat rare liver conditions. The acquisition enriches Rare Disease portfolio of Ipsen, with promising therapeutics for pediatric and adult rare cholestatic-liver diseases, innovative pipeline potential, as well as scientific and commercial capabilities.
In December 2021, ABC Farmaceutici S.p.A. (“ABC”) announced that it has been completely acquired by ICE Pharma, a niche API and specialty ingredients company. This acquisition enhances the product portfolio of ICE Pharma.
Recent Agreement in the Primary Biliary Cholangitis Therapeutics Market
In May 2022, Intercept Pharmaceuticals, Inc. announced that it has entered into an agreement to sell to Advanz Pharma, a pharmaceutical company with a strategic focus on specialty and hospital pharmaceuticals in Europe, certain foreign subsidiaries and rights regarding Intercept’s international operations, including a license to commercialize Ocaliva (obeticholic acid) outside of the U.S.
Recent Product Approval in the Primary Biliary Cholangitis Therapeutics Market
Global pharma major Lupin Limited (Lupin) announced that it has received approval from the U.S. Food and Drug Administration (FDA) for its Abbreviated New Drug Application for Obeticholic Acid Tablets, 5mg and 10mg, a generic equivalent of Ocaliva Tablets, 5 mg, and 10 mg, of Intercept Pharmaceuticals, Inc. This product will be manufactured at Lupin’s Nagpur facility in India.
Recent Partnership in the Primary Biliary Cholangitis Therapeutics Market
In December 2021, Ipsen and Genfit have entered a long-term strategic partnership in which Genfit has given Ipsen exclusive worldwide license to develop, manufacture, and commercialize its experimental late-stage treatment elafibranor, for people living with primary biliary cholangitis. The partnership also gives Ipsen access to future clinical programs led by Genfit and combines scientific expertise and proprietary technologies in liver disease of Genfit with development and commercialization capabilities of Ipsen.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the primary biliary cholangitis therapeutics market analysis from 2022 to 2032 to identify the prevailing primary biliary cholangitis therapeutics market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the primary biliary cholangitis therapeutics market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global primary biliary cholangitis therapeutics market trends, key players, market segments, application areas, and market growth strategies.
Primary Biliary Cholangitis Therapeutics Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 1.4 billion |
| Growth Rate | CAGR of 7.1% |
| Forecast period | 2022 - 2032 |
| Report Pages | 229 |
| By Drug type |
|
| By Distribution Channel |
|
| By Region |
|
| Key Market Players | Sun Pharmaceutical Industries Ltd, GENFIT, ABC Farmaceutici S.p.a., AMAGEN INDIA LIFE SCIENCES, Leeford Healthcare Limited, Alkem Laboratories Ltd., Lupin, Ipsen Pharma, Zydus Lifesciences Limited, Intercept Pharmaceuticals, Inc. |
Analyst Review
According to the insights of the CXOs, the primary biliary cholangitis therapeutics market is poised to grow at a notable pace due to rise in cases of primary biliary cholangitis day by day.
As per the perspectives of CXOs, advancements in primary biliary cholangitis therapeutics, increase in awareness and diagnosis rate of primary biliary cholangitis drive the demand for primary biliary cholangitis therapeutics.The CXOs further added that the demand for new primary biliary cholangitis therapeutics options is on continuous rise due to research and development of primary biliary cholangitis therapeutics, increase in healthcare expenditure and rise in patient advocacy and support. Moreover, emerging markets gain importance for the majority of the primary biliary cholangitis therapeutics manufacturers and distributors, as they focus on unmet demand for treating diseases. Hence, many companies have started introducing advanced products to cater to the needs of a growth in population, especially in developing economies.
North America garnered the highest market share in 2022 and is expected to maintain its lead during the forecast period, in terms of revenue, owing to availability of robust healthcare infrastructure, strong presence of key players, and rise in healthcare expenditure. However, Asia-Pacific is anticipated to witness notable growth owing to increase in use of primary biliary cholangitis therapeutics, high unmet medical demands, presence of high population base, and increase in public–private investments in the healthcare sector.
The primary biliary cholangitis therapeutics market was valued at $683.95 million in 2022 and is estimated to reach $1,360.70 million by 2032, exhibiting a CAGR of 7.1% from 2023 to 2032.
The base year is 2022 in Primary Biliary Cholangitis Therapeutics Market.
The forecast period for Medical Gases and Equipment Market is 2023 to 2032.
The upcoming trends of Primary Biliary Cholangitis Therapeutics Market in the world are rapid advancements in treatment of primary biliary cholangitis; rise in number of research regarding primary biliary cholangitis therapeutics; and increase in number of patients suffering from primary biliary cholangitis.
The primary drug segment generated maximum revenue in 2022, owing to high adoption of primary drug for the treatment of PBC and the effectiveness of primary drug against PBC.
North America was the major revenue contributor in 2022.
The top companies to hold the market share in Primary Biliary Cholangitis Therapeutics are ABC Farmaceutici S.p.a., Alkem Laboratories Limited, AMAGEN INDIA LIFE SCIENCES, GENFIT, Intercept Pharmaceuticals, Inc., Ipsen Pharma, Leeford Healthcare Ltd, Lupin, Sun Pharmaceutical Industries Ltd., and Zydus Lifesciences Limited.
Yes, competitive analysis is included in Primary Biliary Cholangitis Therapeutics Market
Loading Table Of Content...
Loading Research Methodology...


