Internet of Medical Things (IoMT) - Healthcare’s Healing Hand

Internet of Medical Things (IoMT) refers to the network of interconnected medical devices and healthcare apps that use the internet to gather, process, and share health data. To deliver real-time data, improve patient care, and enhance clinical outcomes, this ecosystem consists of wearable technology, smart diagnostic instruments, remote patient monitoring systems, and hospital equipment that communicate with healthcare IT systems. The convergence of IoMT and AI & ML further facilitates continuous patient monitoring, early disease detection, and individualized treatments planning, thereby improving the efficiency and accuracy of healthcare services.
Internet of Medical Things (IoMT), once regarded with skepticism, has now become a prominent trend globally. This shift in perception has increased awareness for IoMT services, which has significantly shaped the growth trajectory of the industry. The outbreak of COVID-19 pandemic served as the key driving force for the adoption and deployment of IoMT devices. Further, alarming need for remote healthcare services and strain on healthcare systems predominantly highlighted the potential of IoMT technology.
Deciphering the Deployment Rate of IoMT Devices
The necessity for remote monitoring services and to efficiently manage healthcare resources have led to substantial increase in the deployment of IoMT devices. According to a study, around 3.2 million IoMT devices were deployed globally in 2021. This number is estimated to reach 7.4 million by 2026. Thus, increasing penetration of this technology will revolutionize the healthcare sector, enhancing diagnostics and treatments. Dr. Eric Topol—Geneticist, Cardiologist, and Digital Medicine Researcher—says “Healthcare is not about what happens to you. It's about how you react to it. The Internet of Medical Things (IoMT) is revolutionizing this reaction, empowering individuals to take control of their health and transform lives.”
The global Internet of Things (IoT) in healthcare industry was valued at $113.75 billion in 2019, and is estimated to reach $332.67 billion by 2027, growing at a CAGR of 13.2% from 2020 to 2027. This growth reflects the rising demand for innovative healthcare solutions, with various organizations and key industry players stepping up to meet this demand. For instance, in April 2021, Hillrom announced significant technological advancements with the launch of the new Welch Allyn PanOptic Plus Ophthalmoscope and the Welch Allyn MacroView Plus Otoscope, which support earlier diagnosis and treatment. Similarly, in May 2023, Medtronic Plc., a healthcare technology company, acquired EOFlow Co. Ltd., the manufacturer of EOPatch devices. With this acquisition, the company aims to expand its existing next-generation continuous glucose monitor and meal detection technology algorithm.
Strong government backing has further facilitated the launch and approval of IoMT devices. In January 2020, JD Health in China launched a "family doctor" telehealth service, which handles an average daily consultation volume of over 120,000 people. Moreover, in February 2024, the European Union announced substantial funding of €67.5 million to accelerate the adoption of AI technologies in healthcare. This funding aims to foster the development and uptake of AI-based healthcare products and services that enhance patient safety and well-being while maintaining privacy and security.
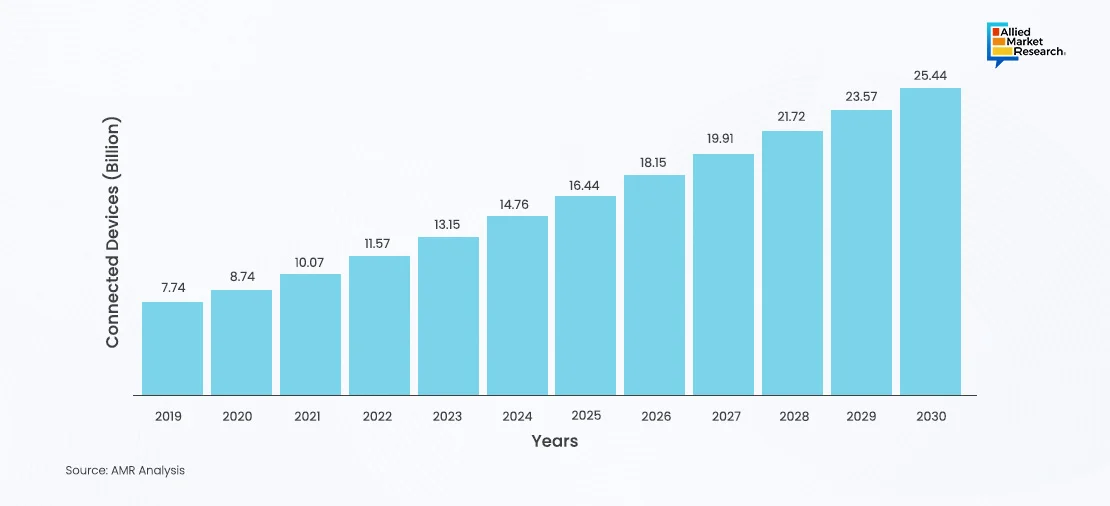
The growing adoption and number of IoMT connected devices worldwide is evident from the steady increase in the number of devices from 2019 to 2030. Starting at 7.74 million in 2019, the number of connected devices was to 8.74 million in 2020 and continued to increase to 10.07 million in 2021, reaching 11.57 million in 2022. This trend persisted, with significant growth each year, culminating in an estimated 25.44 million devices by 2030. This exponential growth highlights the increasing reliance on IoMT for enhancing healthcare delivery and patient outcomes. Thus, the continuous upward trend signifies a robust future for IoMT, driving innovation and efficiency in healthcare.
Key Components of IoMT—Enabling Enhanced Connected Care
Wearable Devices: Wearable devices are portable gadgets that monitor various health metrics, such as heart rate, activity levels, and sleep patterns. Fitness trackers like Fitbit and smartwatches such as the Apple Watch exhibit features like ECG monitoring and fall detection, providing real-time data to both users and healthcare providers. The companies such as Garmin and Samsung also produce advanced wearable devices that integrate with health apps and platforms, enabling continuous health monitoring and personalized insights.
Implantable Devices: Implantable devices are medical devices inserted into the body to monitor and manage various health conditions. These devices can continuously collect and transmit critical health data. Examples include Medtronic’s pacemakers, which regulate heartbeats and can send data to healthcare providers for remote monitoring. Another example is the Dexcom G6 continuous glucose monitor, which is implanted under the skin to provide real-time blood glucose readings for diabetes management. Abbott also manufactures implantable devices such as the FreeStyle Libre continuous glucose monitor system, enhancing chronic disease management through continuous monitoring.
Clinical Devices: Clinical devices are sophisticated tools used in medical settings to monitor, diagnose, and treat patients. These devices are often connected to hospital networks to facilitate data sharing and integration with electronic health records. Examples include GE Healthcare’s connected imaging systems, such as MRI and CT scanners, which provide detailed diagnostic images and integrate with hospital IT systems for comprehensive patient management. Another example is the BD Alaris infusion pump, which ensures precise medication delivery and integrates with hospital monitoring systems to enhance patient safety and treatment accuracy. Philips Healthcare also produces a range of clinical devices, including patient monitoring systems and diagnostic equipment, that contribute to efficient and effective clinical care.
Regulatory Scenario of IoMT
The regulatory landscape of the IoMT involves multiple agencies and organizations to ensure the safety, effectiveness, and interoperability of medical devices. Key organizations in this regulatory scenario include the U.S. Food and Drug Administration (FDA), European standards organizations, the Office of the National Coordinator for Health Information Technology (ONC), the International Organization for Standardization (ISO), and the International Electrotechnical Commission (IEC).
Food and Drug Administration (FDA): The U.S. FDA is pivotal in regulating IoMT devices in the U.S. It classifies medical devices into three classes based on their risk to patients. Class I devices are low risk, while Class III devices are high risk and require the most stringent regulatory controls. The FDA's premarket approval (PMA) process and 510(k) clearance are critical pathways for the approval of these devices. For instance, Medtronic's MiniMed 670G insulin pump, a Class III device, underwent rigorous evaluation to ensure its safety and effectiveness for diabetes management. Additionally, the FDA has issued guidelines for software-as-a-medical-device (SaMD) to address the growing role of software in medical technology. Cybersecurity is another focus area, with the FDA providing detailed guidelines to protect against cyber threats, crucial for the safety of connected medical devices. For instance, in February 2020, GE Healthcare company, introduced a new cybersecurity service offering that brings together medical device expertise, AI, and process management tools to help hospital groups in their fight against cybersecurity threats.
European Standards Organizations: In the European Union, medical devices are regulated under the Medical Devices Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). These regulations ensure that devices marketed in the EU meet high standards of safety and performance. The CE marking process, managed by Notified Bodies, signifies compliance with MDR or IVDR requirements. Siemens Healthineers, for example, markets its devices with CE marking, indicating adherence to these stringent regulations. The European Database on Medical Devices (EUDAMED) enhances transparency and traceability, making detailed information on medical devices available to stakeholders. Additionally, the MDR includes provisions for cybersecurity and data protection, essential for the IoMT security of devices.

ONC: The Office of the National Coordinator for Health Information Technology (ONC) is a key entity in the U.S. responsible for advancing health IT and ensuring the interoperability of health information. The ONC's Health IT Certification Program ensures that electronic health records and other health IT products meet specific standards. This includes interoperability standards that are critical for the integration of IoT devices in healthcare systems. The ONC’s Trusted Exchange Framework and Common Agreement (TEFCA) aims to create a universal floor for interoperability across health information networks. For instance, ONC supports initiatives that enable the seamless exchange of data from wearable devices like Apple Watch, which can integrate health data into electronic health record systems.
ISO and IEC: ISO and IEC develop international standards to ensure the safety, quality, and interoperability of medical devices. ISO 13485, for instance, specifies requirements for a quality management system for medical devices, and is widely adopted by manufacturers such as Philips Healthcare to ensure product quality. IEC 62304 provides a framework for the lifecycle processes of medical device software, ensuring their safety and reliability. This is particularly important for complex IoMT systems where software plays a critical role. ISO/IEC 27001 sets criteria for an information security management system, crucial for protecting the vast amounts of data generated by IoMT devices. For example, GE Healthcare adheres to these standards to safeguard data from their connected imaging devices.
Thus, collectively, these organizations create a robust regulatory framework to ensure IoMT devices are safe, reliable, and effective, while efficiently facilitating their integration into healthcare systems. Their collaborative efforts help to advance medical technology while protecting patient safety and ensuring high standards of healthcare delivery.
Peering into the Pain Points
Despite the complex nature of IoMT devices, they are highly vulnerable to cyber-attacks due to lack of in-built security features, misconfigurations in operating systems, and unsecured communication protocols. The findings by the Ponemon Institute revealed that IoMT and IoT devices are the primary targets of cyber attackers. IoMT devices were responsible for 21% of all ransomware attacks. Estimates by Check Point Research indicated that the healthcare sector was the most vulnerable to cyberattacks among all sectors in India, with an organization being attacked 1,866 times per week on an average. The study further revealed that there was an increase of 38% in cyberattacks in 2022 as compared to 2021.
Thus, rising risk of cyberattacks highlights the need to deploy robust security measures within the healthcare sector to help tackle such attacks. To ensure enhanced cybersecurity, devices must undergo critical assessment and strictly adhere to regulatory compliances to prevent unauthorized access to hackers, safeguard data privacy, and maintain data integrity.
Screening the Success Stories of Real-world IoMT Devices
Smart Pill Dispensers: Pillsy is a smart pill bottle that connects to the phone via Bluetooth, tracks medication schedules, sends reminders to take medication on time, which is displayed on a bottle cap with a built-in digital timer. Studies reveal that a common reason why patients do not adhere to the medication schedule is forgetfulness. According to recent estimates, noncompliance costs the U.S. healthcare systems around $289 billion each year, thus leading to approximately 10% of hospitalizations.
Another smart system that helps to follow treatment plans is Pillo Health, which is integrated with a virtual assistant and a pill dispenser. It provides users with the necessary information about medicines, reminds them to take pills on time, and facilities remote monitoring by enabling users to connect with their doctors or caregivers.
Remote Monitoring Devices: Dexcom CGM system is a device that constantly checks sugar level, sends alerts on high or low levels, and facilitates remote monitoring with caregivers or doctors for treatment plans.
Philips respiratory devices monitor breathing issues such as chronic obstructive pulmonary disease and sleep apnea from a distance. Philips’ devices, including oxygen concentrators and CPAP devices monitor oxygen levels of patients and collect & send data about how patients breathe, which help doctors to plan treatment regimens and keep track of how treatments are working.
Thus, real-world IoMT devices are transforming healthcare by improving diagnoses, monitoring treatment routines, and increasing the efficiency of healthcare services, thus enhancing patient outcomes.
Way Ahead
IoMT acts as a transformative force in healthcare, leveraging interconnected devices and advanced technologies to enhance patient care and clinical outcomes. It serves as a next-generation bio-analytical tool, which combines network-linked biomedical devices with relevant software applications for improving human health. With increasing adoption globally, IoMT is poised to revolutionize healthcare delivery. The regulatory bodies such as FDA, European Standardization Organizations, ONC, ISO, and IEC play crucial roles in ensuring the safety, efficacy, and interoperability of IoMT devices. As the IoMT ecosystem continues to evolve, future opportunities abound. These include advancements in personalized medicine, AI-driven diagnostics, and remote patient monitoring, leading to more precise treatments and improved patient outcomes. Moreover, the integration of IoMT with telehealth services and predictive analytics holds promise for proactive healthcare management and cost savings. Embracing these opportunities will drive innovation, improve healthcare accessibility, and ultimately, enhance the quality of life for individuals worldwide.
Allied Market Research (AMR) highlights the potential of IoMT in transforming the healthcare landscape. AMR’s commitment to provide comprehensive market insights, trends, and forecasts enables vendors to make informed decisions that drive growth and success. Through tailored reports, custom consulting services, and ongoing support, AMR equips vendors with the knowledge and tools needed to capitalize on the inherent advantages of IoMT. Whether identifying emerging trends, evaluating market entry strategies, or optimizing product portfolios, our team of experts provides actionable insights to help your business reach its goal. For more details, contact our esteemed professionals today!



