Amniotic Product Market Research, 2033
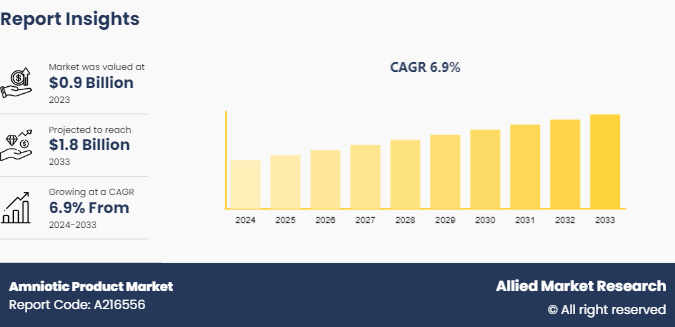
The global amniotic product market size was valued at $0.9 billion in 2023, and is projected to reach $1.8 billion by 2033, growing at a CAGR of 6.9% from 2024 to 2033. The amniotic product market growth is driven by several factors, including their effectiveness in promoting tissue regeneration and reducing inflammation, which is particularly beneficial for treating chronic wounds and orthopedic conditions.
Market Introduction and Definition
Amniotic products, derived from the amniotic membrane and fluid of the placenta, are used in regenerative medicine due to their rich content of growth factors, cytokines, and extracellular matrix components. These products, available as grafts, patches, injections, and topical solutions, promote tissue repair, reduce inflammation, and prevent scarring. The mechanism of action involves delivering bioactive molecules that enhance cellular migration, proliferation, and differentiation, leading to accelerated healing and reduced inflammation. Common applications include wound healing, ophthalmology, orthopedics, and plastic surgery, making amniotic products versatile tools in improving patient outcomes in various medical field.
Key Takeaways
- The amniotic product market share study covers 20 countries. The research includes a segment analysis of each country in terms of value ($Billion) for the projected 2024-2033 amniotic product market forecast period.
- More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major amniotic product industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrated high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and to assist stakeholders in making educated decisions in order to achieve their most ambitious growth objectives.
Key Market Dynamics
One key driver is the increasing prevalence of chronic diseases such as diabetes and cardiovascular disorders, which often lead to conditions like diabetic ulcers and osteoarthritis. These conditions require advanced wound care and regenerative therapies, where amniotic products excel due to their rich source of growth factors and cytokines that aid in healing.
However, market growth faces restraints such as regulatory challenges and variability in product quality and efficacy. Regulatory approvals can be stringent and vary across regions, which can hinder market entry and expansion. Moreover, ensuring consistent product quality and demonstrating efficacy through clinical trials are ongoing challenges for manufacturers in this sector.
Despite these challenges, the amniotic products market presents significant opportunities. One major opportunity lies in expanding applications beyond traditional wound care to include new therapeutic areas such as ophthalmology, where amniotic membranes are used to treat conditions like corneal defects and dry eye syndrome. Additionally, advancements in biotechnology and regenerative medicine offer opportunities for developing enhanced formulations and delivery methods for amniotic products, improving their efficacy and patient outcomes.
Moreover, increasing awareness among healthcare professionals and patients about the benefits of regenerative therapies is driving market growth. As more physicians and patients recognize the potential of amniotic products in enhancing healing and reducing recovery times, demand is expected to rise. Collaborations between academic institutions, healthcare providers, and industry players also present opportunities for advancing research and expanding market reach globally.
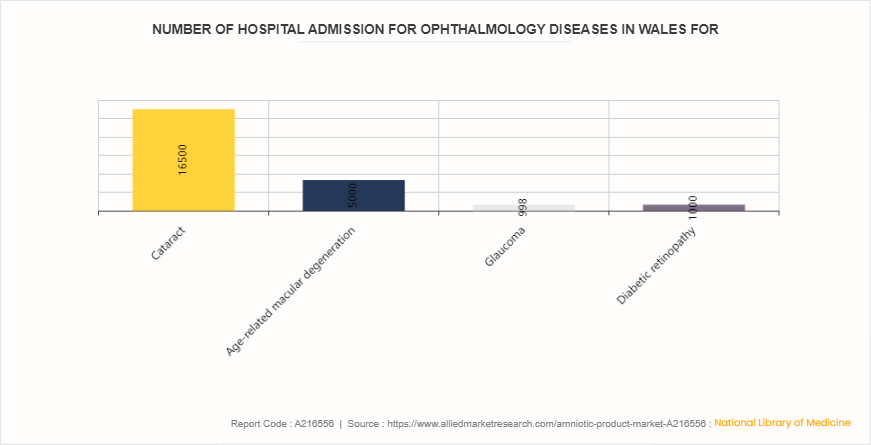
Number of hospital admission for ophthalmology diseases in Wales for Amniotic products Market
According to the data published by Welsh government under Eye care statistics: April 2021 to March 2023. Hospital admissions for various ophthalmology conditions were notable: 16, 500 cases of cataract, 5, 000 for age-related macular degeneration, 998 for glaucoma, and 1, 000 for diabetic retinopathy. These figures highlight significant patient volumes requiring medical intervention, potentially indicating a robust market demand for amniotic products in treating these conditions. The data underscores the substantial need for effective therapies and treatments in the ophthalmology sector, suggesting a promising market opportunity for advanced medical solutions like amniotic products.
Market Segmentation
The amniotic product industry is segmented into product, application, end user and region. On the basis of product, the market is categorized into cryopreserved amniotic, and lyophilization amniotic. On the basis of application, the market is segmented into surgical wounds, ophthalmology, orthopedic, and others. On the basis of end user, the market is segmented into hospitals, ambulatory surgical centers, specialized clinics, and research centers & laboratory. Region wise, it is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional/Country Market Outlook
The amniotic product market size shows promising growth globally, with North America leading due to advanced healthcare infrastructure and high adoption rates. Europe follows, driven by increasing chronic wound cases and aging populations. In Asia-Pacific, countries like China and India are experiencing rapid growth due to rising healthcare investments and awareness. Latin America and the Middle East & Africa are emerging markets, with improving healthcare access and demand for advanced medical treatments boosting market expansion. Overall, regional market dynamics reflect varying levels of healthcare development, regulatory landscapes, and demographic trends influencing the adoption and growth of amniotic products.
- According to an article published by National Library of Medicine in 2024, stated that the annual incidence of corneal ulcers in the U.S. alone is estimated to be between 30, 000 and 75, 000.
Industry Trends
- In October 2022, Membrane Wrap – Hydro, a hydrated human amnion membrane allograft by BioLab Sciences, has been recognized by the Food and Drug Administration’s (FDA) Tissue Reference Group (TRG) as a human cell, tissue, or cellular or tissue-based product (HCT/P) . This recognition may bring Membrane Wrap – Hydro one step closer to streamlining the reimbursement process for providers.
Competitive Landscape
The major players operating in the amniotic product market shareinclude Allosure, Inc., Amnio Technology, LLC, Applied Biologics LLC, FzioMed, Inc., Human Regenerative Technologies, LLC, Integra Lifesciences Holdings Corporation, Corza Ophthalmology, MiMedx Group, Inc., Skye Biologics Inc., Tissue-Tech, Inc. Other players in the Amniotic products market include Thea Pharma, Merakris Therapeutics, and so on.
Recent Key Strategies and Developments
- In August 2021, Vivex Biologics, Inc., a leading regenerative medicine company specializing in the development of naturally sourced treatments, announced the launch of CYGNUS Matrix Disks, the latest configuration from the CYGNUS family of amniotic tissue allografts.
- In January 2022, Laboratoires Thea SAS, one of the leading independent pharmaceutical companies in Europe, signed an agreement to purchase seven branded ophthalmic products from Akorn Operating Company LLC. The acquisition includes AcellFX (acellular amniotic membrane) , which provides a protective environment or covering for repair to the ocular surface.
Key Sources Referred
- World Health Organization (WHO)
- Centers for Medicare & Medicaid Services (CMS)
- National Health Service (NHS)
- Welsh government
- National Health Mission (NHM)
- Centers for Disease Control and Prevention (CDC)
- Food and Drug Administration (FDA)
- National Institutes of Health (NIH)
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the amniotic product market analysis from 2024 to 2033 to identify the prevailing amniotic product market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the amniotic product market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global amniotic product market trends, key players, market segments, application areas, and market growth strategies.
Amniotic Product Market Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 1.8 Billion |
| Growth Rate | CAGR of 6.9% |
| Forecast period | 2024 - 2033 |
| Report Pages | 216 |
| By Product |
|
| By Application |
|
| By End User |
|
| By Region |
|
| Key Market Players | Tissue-Tech, Inc., Skye Biologics Inc., Integra Lifesciences Holdings Corporation, Allosure, Inc., applied biologics llc, Amnio Technology, LLC, Human Regenerative Technologies, LLC, MiMedx Group, Inc., FzioMed, Inc., Corza Ophthalmology |
The total market value of Amniotic Product Market is $0.9 billion in 2023.
The forecast period for Amniotic Product Market is 2024 to 2033
The market value of Amniotic Product Marketin 2033 is $1.7 billion
The base year is 2023 in Amniotic Product Market.
Top companies such as Allosure, Inc., Amnio Technology, LLC, Applied Biologics LLC, FzioMed, Inc., held a high market position in 2023. These key players held a high market postion owing to the strong geographical foothold in North America, Europe, Asia-Pacific, LAMEA.
The Cryopreserved segment is the most influencing segment in Amniotic Product Market
Loading Table Of Content...


