Companion Diagnostics Market Statistics, 2026
The global companion diagnostics market generated $1,678 million in 2018, and is projected to reach $6,452 million by 2026, growing at a CAGR of 18.3% from 2019 to 2026. A companion diagnostic is an in vitro medical device that provides information which is essential for the safe and effective use of a corresponding drug or biological product. This diagnostic test helps a health care professional to determine whether a particular therapeutic product is beneficial to patients and can outweigh any potential serious side effects or risks.
The companion diagnostics market is expected to experience significant growth during the forecast period owing to surge in R&D of targeted therapies, rise in demand for personalized medicine with increase in awareness in emerging economies, discovery of new biomarkers for various conditions, and higher number of unmet needs for the treatment of cancer are majorly driving the growth of the global companion diagnostics market.
Companion Diagnostics Market Segmentation
The global companion diagnostics market is segmented based on technology type, indication, and region. Based on technology type, the market is classified as immunohistochemistry, polymerase chain reaction (PCR), next generation sequencing (NGS), in situ hybridization, and others. Based on indication, the market is classified as oncology, neurology, and others. The oncology segment is further segmented into lung cancer, colorectal cancer, breast cancer, blood cancer, and others. Based on region, the market is studied across North America (U.S., Canada, and Mexico), Europe (Germany, France, the UK, and rest of Europe), Asia-Pacific (China, Japan, Australia, and rest of Asia-Pacific), and LAMEA (Brazil, South Africa and rest of LAMEA).
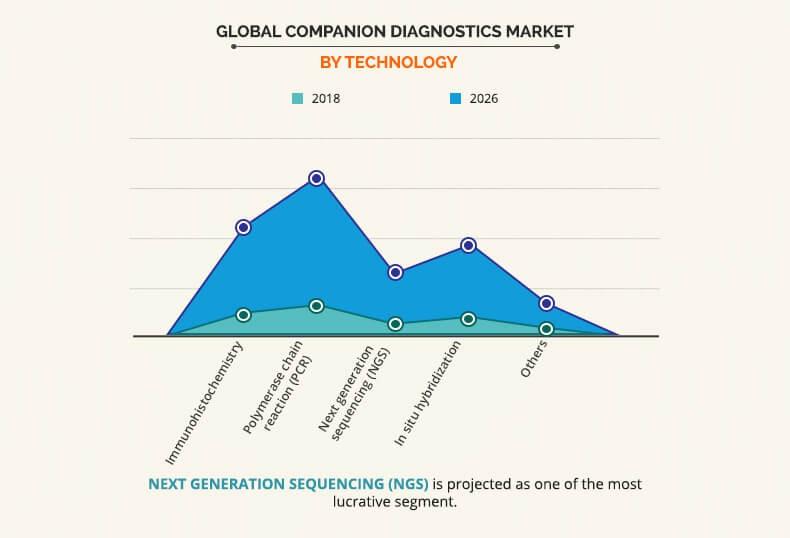
By Technology
Based on technology, the PCR segment dominates the global market, and is anticipated to continue this trend during the forecast period. Some key factors that drive the market growth are cost effectiveness, high sensitivity, and specificity and can be used for simple automated platforms. Furthermore, the sequencing of unknown etiologies of many diseases can be determined by the PCR, which is another key driver of this segment. However, the next generation sequencing segment is estimated to experience rapid growth during the forecast period owing to its key advantages such as higher sensitivity to detect low-frequency variants, lower turnaround time for high sample volumes, ability to sequence hundreds to thousands of genes or gene regions simultaneously etc. are majorly driving the market growth.Oncomine Dx Target Test, FoundationOne CDx, FoundationFocus CDxBRCA Assay and others use next generation sequencing for detection.
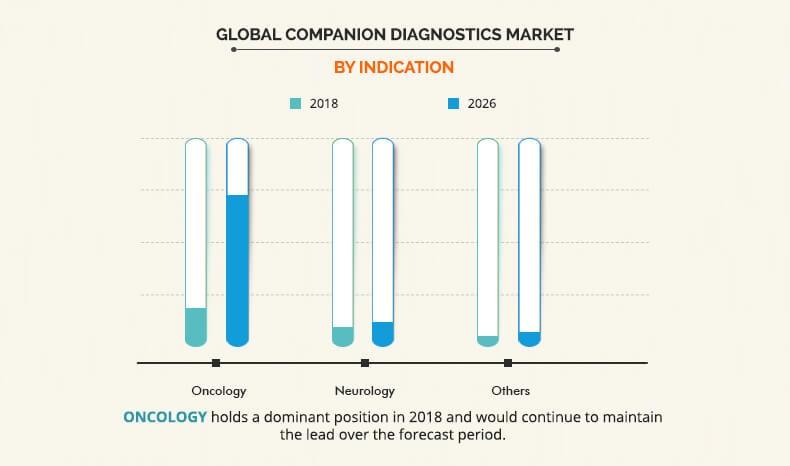
By Indication
Based on indication, the global companion diagnostics market is segmented into oncology, neurology, and others. At present, the oncology segment is the major revenue generating segment and is estimated to experience significant growth during the forecast period. Some key factors such as higher prevalence of cancer, rise in number of R&D activities for cancer, higher number of unmet needs for treatment of cancer, rise in number of FDA approved companion diagnostics, and increasing awareness among the patients about personalized medicine are majorly driving the growth of the oncology segment.
Research in cancer has identified significant differences in gene sequence and expression patterns that can be the basis for targeted therapy. Furthermore, many biomarkers have been identified for different cancers, for which companion diagnostics have been developed. Companion diagnostics are widely used in different forms of cancer such as lung cancer, colorectal cancer, breast cancer, blood cancer, which are the major cause of the death. The companion diagnostics market share is higher for breast cancer as there are many companion diagnostics developed for detection of breast cancer mutations; with large number of target population.
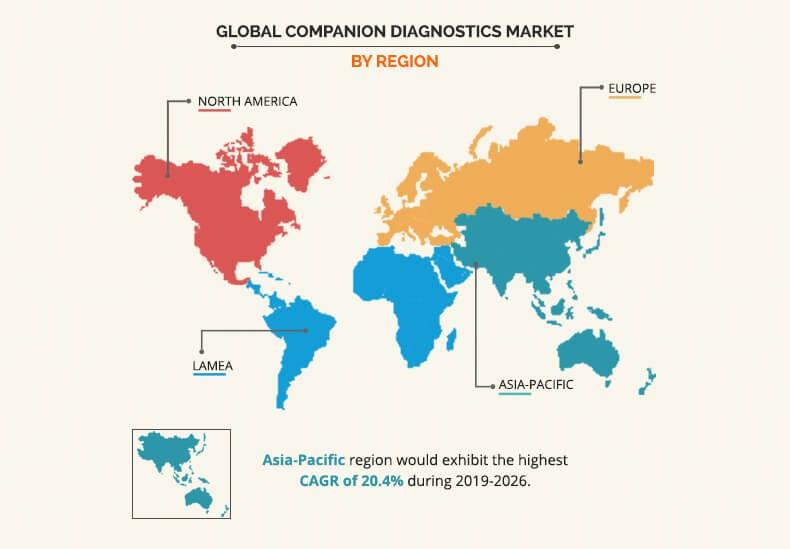
Snapshot of Asia-Pacific Companion Diagnostics Market
Asia-Pacific presents lucrative opportunities for the key players operating in the companion diagnostics market, owing to high population base, growth in awareness about companion diagnostics, development in healthcare infrastructure, and increase in demand for advance therapies. However, higher costs incurred in R&D to developed companion diagnostics can hamper the companion diagnostics market growth in Asia-Pacific.
Competitive Analysis
The key players profiled in this report include Abbott Laboratories Molecular, Inc., Agilent (Dako Denmark A/S), ARUP Laboratories, Inc., BioMerieux SA, Danaher Corporation (Leica Microsystems), Foundation Medicine, Inc., Myriad Genetics, Inc., Qiagen N.V., Roche (Ventana Medical Systems, Inc.), and Thermo Fisher Scientific (Life Technologies Corporation).
Key Benefits for Companion Diagnostics Market
- The study provides an in-depth analysis of the companion diagnostics market size along with the current trends and future estimations to elucidate the imminent investment pockets.
- It offers a quantitative analysis from 2018 to 2026, which is expected to enable the stakeholders to capitalize on the prevailing market opportunities.
- A comprehensive companion diagnostics market analysis of all the regions is provided to determine the prevailing opportunities.
- The profiles and growth strategies of the key players are thoroughly analyzed to understand the competitive outlook of the global market.
Companion Diagnostics Market Report Highlights
| Aspects | Details |
| By Technology Type |
|
| By Indication |
|
| By Region |
|
| Key Market Players | Danaher Corporation (Leica Microsystems), Agilent (Dako Denmark A/S), QIAGEN N.V., Abbott Laboratories Molecular, Inc., Thermo Fisher Scientific (Life Technologies Corporation), Myriad Genetics, Inc., Foundation Medicine, Inc., BioMerieux, ARUP Laboratories, Inc., Roche (Ventana Medical Systems, Inc) |
Analyst Review
Companion diagnostics is a medical device, often an in vitro device, that provides information essential for the safe and effective use of a corresponding drug or biological product. This test helps a health care professional to determine patients that can benefit from a particular therapeutic product, and helps identify patients at increased risk of serious adverse reactions. The adoption of companion diagnostics is expected to increase due to surge in adoption of personalized medicine.
Rise in demand for advanced therapies, growth in R&D activities to develop low cost & highly efficient drugs, increase in awareness in personalized medicines, higher number of unmet needs for treatment of cancer, surge in number of target population and rise in demand for cost effective diagnosis are some key factors that majorly driving the market growth.
North America is expected to remain dominant during the forecast period due to higher adoption of advanced therapies, rise in demand for companion diagnostics, presence of key players, and higher prevalence of cancer. In addition, Asia-Pacific and LAMEA are expected to offer lucrative opportunities to the key players during the forecast period due to developments in healthcare infrastructure with a rise in demand for personalized medicine.
The total market value of Companion Diagnostics market is $ 1,678 million in 2018.
The forcast period for Companion Diagnostics market is 2019 to 2026
The market value of Companion Diagnostics market in 2026 is $ 6,452 million.
The base year is 2018 in Companion Diagnostics market
Top companies such as, Abbott Laboratories Molecular, Inc., Agilent (Dako Denmark A/S), ARUP Laboratories, Inc., BioMerieux SA, Danaher Corporation (Leica Microsystems), Foundation Medicine, Inc., Myriad Genetics, Inc., Qiagen N.V., Roche (Ventana Medical Systems, Inc.), and Thermo Fisher Scientific (Life Technologies Corporation) held a high market position in 2018. These key players held a high market position owing to the strong geographical foothold in different regions.
The polymerase chain reaction (PCR) segment dominates the global market, and is anticipated to continue this trend during the forecast period.
Higher costs incurred in R&D to developed companion diagnostics can hamper the companion diagnostics market growth in Asia-Pacific.
Companion diagnostic is an in-vitro diagnostic process that provides information about the therapeutic response of a patient for a specific treatment. Companion diagnostic is a form of personalized medicine that includes drug-testing, therapies, clinical trials, and research.
Loading Table Of Content...