D-dimer Testing Market Research, 2032
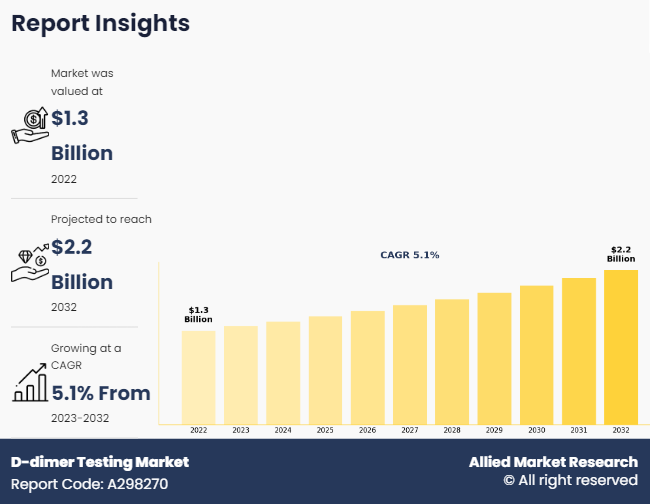
The global d-dimer testing market size was valued at $1.3 billion in 2022, and is projected to reach $2.2 billion by 2032, growing at a CAGR of 5.1% from 2023 to 2032. The rise in incidents of blood clot disorder among individuals is the major factor driving the growth of d-dimer testing. According to World Federation of Hemophilia, as of July 2022, 10,276 people with hemophilia were enrolled from 87 hemophilia treatment centers in 40 countries.
The D-dimer test is vital in diagnosing and monitoring various blood clotting disorders, including deep vein thrombosis (DVT), pulmonary embolism (PE), disseminated intravascular coagulation (DIC), and stroke. DVT, characterized by blood clots forming deep within veins, commonly affects the lower limbs but can occur elsewhere in the body. PE occurs when a clot dislodges and travels to the lungs, often originating from a DVT. DIC, a severe condition marked by excessive clot formation throughout the body, can result from factors like inflammation, infection, or cancer, leading to organ damage and other serious complications.
In addition, the D-dimer test aids in assessing the efficacy of treatments for disorders such as DIC. By measuring levels of D-dimer, a protein fragment produced when blood clots dissolve, the test provides valuable insights into the likelihood of clot formation. Hence, the D-dimer test serves as a crucial diagnostic and monitoring tool, enabling healthcare professionals to initiate prompt interventions and ensure effective management of blood clotting disorders, thereby mitigating the risk of life-threatening complications.
Market Dynamics
Detailed research across various medical disciplines has highlighted the major role of elevated D-dimer levels as a significant biomarker in several clinical conditions. From atrial fibrillation to HIV infection, coronary artery disease, and acute pancreatitis, the prognostic value of D-dimer testing has emerged as indispensable in modern healthcare diagnostics. Atrial fibrillation, a common cardiac arrhythmia, often presents challenges in diagnosis and management due to its diverse clinical manifestations. However, elevated D-dimer levels have been identified as a reliable indicator of thrombotic events associated with this condition, aiding clinicians in timely intervention and risk stratification strategies. Similarly, in the context of HIV infection, D-dimer elevation serves as a crucial marker of increased coagulation and endothelial dysfunction, offering insights into disease progression and complications such as thromboembolic events.
Moreover, in the condition of cardiovascular health, higher D-dimer levels have been closely linked with coronary artery disease, serving as a predictor of adverse cardiovascular events and guiding therapeutic decisions. In addition, in acute pancreatitis, where early identification of complications is paramount, D-dimer testing emerges as a valuable tool in assessing the extent of vascular involvement and predicting disease severity. As these research findings continue to pass clinical practice, the D-dimer testing market growth is projected to increase, driving growth in diagnostic laboratories and prompting further innovations in assay technologies. Binding the predictive power of D-dimer levels not only enhances patient care but also highlights the role of laboratory medicine in modern healthcare models.
The lack of standardization among D-dimer assays and testing methodologies poses a significant obstacle to the comparability of results, thereby impeding the accurate clinical interpretation of these tests. This disparity not only complicates the evaluation of patients but also undermines the reliability of diagnostic conclusions. The inconsistencies arising from varied testing procedures hinder the ability of healthcare professionals to confidently assess patient conditions, potentially leading to improper diagnoses or delayed treatments. Moreover, the absence of uniformity in D-dimer testing methodologies obstructs efforts to establish consistent guidelines for clinical practice, further worsening the problem. This fragmentation within the D-dimer testing industry not only deviates medical decision-making but also limits advancements in diagnostic approaches and treatment strategies. Consequently, the hampers D-dimer testing market forecast, crucial for effective management of thrombotic disorders and other medical conditions.
The emergence of aptamers as an alternative to antibodies for D-dimer testing indicates a major shift in diagnostic methodologies, offering a cost-effective and enduring solution. Aptamers, with their differentiating attributes, present an attractive prospect for revolutionizing D-dimer testing. Their affordability, stability, and expedited production process differentiate them from the traditional employment of antibodies. Notably, aptamers boast a prolonged shelf life, further enhancing their appeal in the medical landscape. The cost-effectiveness of aptamers not only reduces financial burdens on healthcare systems but also expands D-dimer testing market opportunity, particularly in resource-limited settings. Moreover, their stability ensures consistent performance, mitigating concerns regarding assay variability often associated with antibodies. The swifter production cycle of aptamers accelerates testing protocols, facilitating prompt diagnosis and subsequent patient management.
In addition to their functional control, aptamers offer a sustainable solution by diminishing the reliance on antibodies, thereby mitigating supply chain vulnerabilities and reducing dependence on animal-derived reagents. This transition not only aligns with ethical considerations but also fosters environmental sustainability and would support in growth of D-dimer testing market size.
Segments Overview
The D-dimer testing market share is segmented on the basis of test type, application, end-use, and region. By test type, the market is classified into laboratory tests and point-of-care tests. By application the market is divided into deep vein thrombosis (DVT), pulmonary embolism (PE), disseminated intravascular coagulation (DIC), and others. By end-use, the market is classified into hospitals, academic & research institutes, diagnostic centers, and others. By region, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
By Test Type
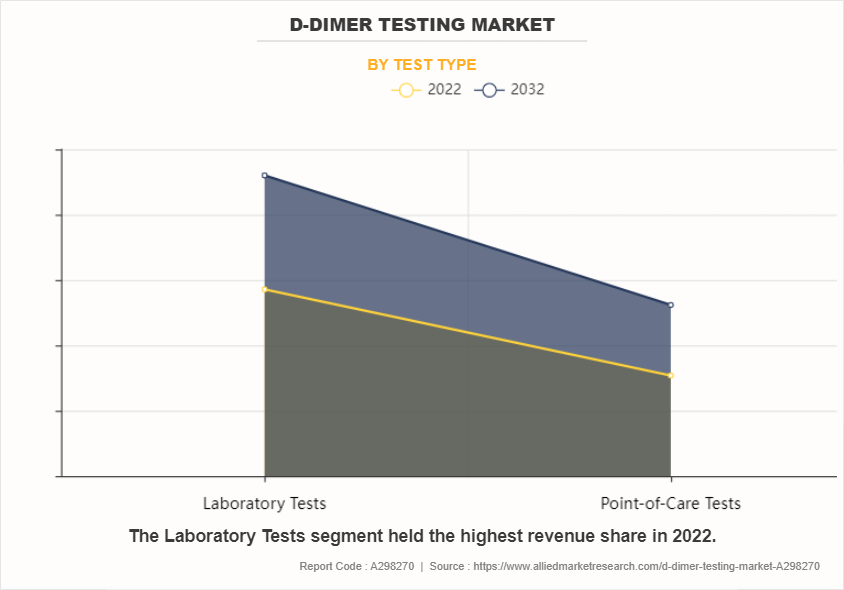
By test type, the laboratory tests sub-segment dominated the global D-dimer testing market in 2022. Within this sub-segment, the significance of D-dimer testing stands out prominently, reflecting its criticalness in assessing various medical conditions, particularly those related to thrombosis and clotting disorders. The prevalence of laboratory-based D-dimer tests highlight their efficacy and reliability in providing accurate diagnostic insights. Such tests offer clinicians with important information regarding the presence of fibrin degradation products, aiding in the timely detection and management of thrombotic events. Moreover, their widespread adoption within clinical settings emphasizes about their indispensable role in modern healthcare practices.
By Application
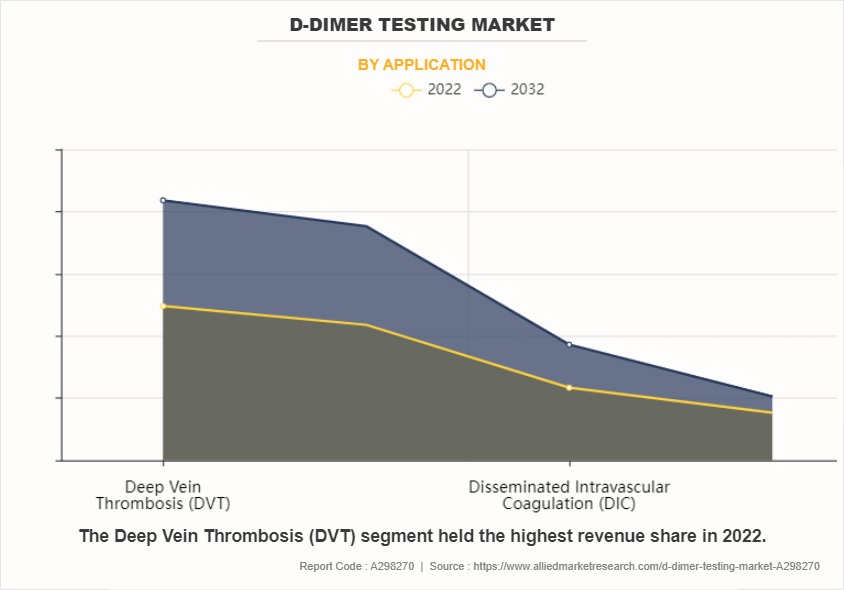
By application, the deep vein thrombosis (DVT) sub-segment dominated the global D-dimer testing market in 2022. This sub-segment's prominence highlights the critical role D-dimer testing plays in diagnosing and managing DVT, a condition characterized by blood clot formation in the deep veins, often within the legs. As a non-invasive diagnostic tool, D-dimer testing serves as a frontline method for assessing the likelihood of thrombotic events, including DVT, by detecting higher levels of D-dimer, a fibrin degradation product indicative of ongoing fibrinolysis. Its utility extends beyond diagnosis, guiding treatment decisions and monitoring therapeutic efficacy, particularly in the context of anticoagulant therapy. The prevalence of DVT cases, along with the rising awareness regarding thromboembolic disorders and the need for timely intervention, further boosts the demand for D-dimer testing within the DVT sub-segment.
This dominance not only reflects the clinical significance of DVT but also the major role played by D-dimer testing in enhancing diagnostic accuracy, optimizing patient care, and mitigating the risk of thrombotic complications.
By End-use
By end-use, the hospitals sub-segment dominated the global D-dimer testing market in 2022. The segment’s dominance highlights the major role hospitals play in diagnostic procedures and healthcare delivery worldwide. Within the complex structure of medical institutions, hospitals stand as primary hubs where patients seek care and clinicians make critical decisions. The dominance of hospitals in D-dimer testing reflects their central position in managing a diverse array of medical conditions, ranging from routine check-ups to emergency interventions. As frontline entities in healthcare, hospitals possess the infrastructure, expertise, and resources necessary for accurate and timely diagnostic testing. Their widespread presence ensures accessibility to D-dimer assays, enabling healthcare professionals to swiftly assess patients' coagulation status and make informed clinical judgments. Moreover, hospitals serve as epicenters of medical innovation and research, continuously advancing diagnostic methodologies to enhance patient care and outcomes.
The prevalence of D-dimer testing within hospital settings highlights its significance in evaluating thrombotic and hemorrhagic disorders, aiding in the prompt diagnosis and management of various medical conditions.

By Region
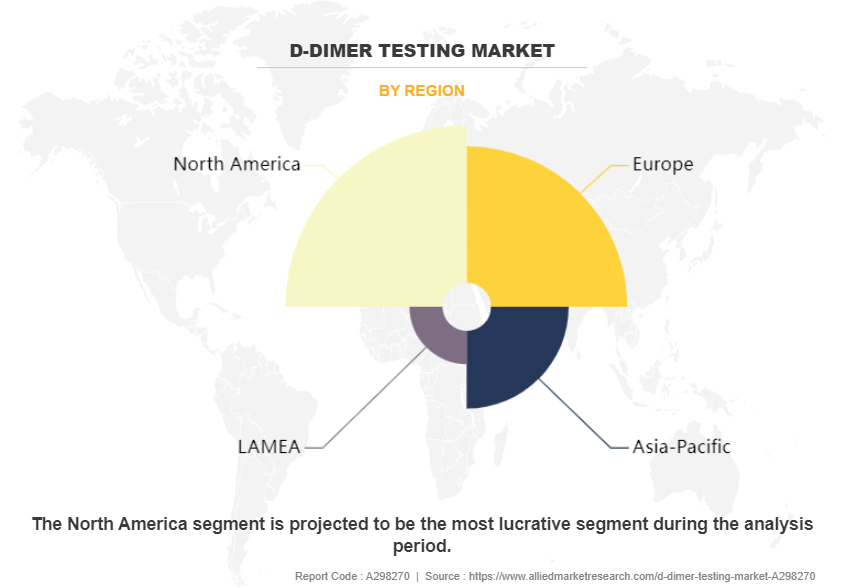
By region, North America dominated the D-dimer testing market in 2022. D-dimer testing has emerged as a dominant diagnostic tool, reflecting the region's emphasis on proactive healthcare management. With its widespread adoption across medical facilities, D-dimer testing has become integral in evaluating thrombotic conditions and ruling out serious clotting disorders. This dominance highlights North America's commitment toward advanced medical practices and its proactive approach to healthcare. The prevalence of D-dimer testing signifies the region's prioritization of early detection and intervention in thrombotic events, aligning with its broader healthcare ethos of preventative care. North America's D-dimer testing market share highlights its role as a major region in innovative medical technologies and diagnostic methodologies, setting a benchmark for other regions. This trend not only reflects the region's investment in advanced medical research and technology but also its dedication to improving patient outcomes through early detection and effective treatment strategies.
Competitive Analysis
The key players profiled in D-dimer testing industry report are Thermo Fisher Scientific Inc., F. Hoffmann-La Roche Ltd., Siemen Healthineers, Abbott, Biomerieux SA, WERFEN, HORIBA, Ltd., Quidel Corporation, Diazyme Laboratories, Inc., and Sekisui Diagnostics.
Product launch and strategic partnership are common strategies followed by major market players. For instance, in June 2022, LumiraDx Limited broadened its cardiovascular diagnostic capabilities by obtaining CE Marks for two pivotal tests on its platform. The newly approved NT-proBNP test targets aiding in the diagnosis of congestive heart failure (CHF), empowering clinicians to assess patients presenting symptoms of CHF swiftly and accurately. In addition, the updated CE Mark for the D-Dimer test enhances its utility to now exclude venous thromboembolism (VTE) in symptomatic patients. This expansion enables healthcare professionals to effectively rule out deep vein thrombosis (DVT) and pulmonary embolism (PE) by employing the LumiraDx D-Dimer test alongside a clinical pre-test probability assessment model. These advancements signify a significant leap in point-of-care diagnostics, offering timely and precise evaluations of cardiovascular conditions. By integrating these advanced tests into their practice, clinicians can expedite diagnostic processes, leading to improved patient outcomes and enhanced management of both CHF and VTE at the point of care.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the d-dimer testing market analysis from 2022 to 2032 to identify the prevailing d-dimer testing market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the d-dimer testing market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global d-dimer testing market trends, key players, market segments, application areas, and market growth strategies.
D-dimer Testing Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 2.2 billion |
| Growth Rate | CAGR of 5.1% |
| Forecast period | 2022 - 2032 |
| Report Pages | 310 |
| By Application |
|
| By End-use |
|
| By Test Type |
|
| By Region |
|
| Key Market Players | Quidel Corporation, biomerieux SA, Abbott, Diazyme Laboratories, Inc., Sekisui Diagnostics, Thermo Fisher Scientific Inc., Horiba Ltd, Siemen Healthineers, F. Hoffmann-La Roche Ltd., WERFEN |
The global D-dimer testing market is experiencing significant growth attributed to the rising demand for Point-of-Care D-dimer testing.
The major growth strategies adopted by the D-dimer testing market players are product launches and partnership agreements.
North America will provide more business opportunities for the global D-dimer testing market in the future.
Thermo Fisher Scientific Inc., F. Hoffmann-La Roche Ltd., Siemen Healthineers, Abbott, Biomerieux SA, WERFEN, HORIBA, Ltd., Quidel Corporation, Diazyme Laboratories, Inc., and Sekisui Diagnostics are the major players in the D-dimer testing market.
The laboratory tests sub-segment of the test type segment acquired the maximum share of the global D-dimer testing market in 2022.
Hospitals are the major customers in the global D-dimer testing market.
The report provides an extensive qualitative and quantitative analysis of the current trends and future estimations of the global D-dimer testing market from 2022 to 2032 to determine the prevailing opportunities.
The increasing recognition of D-dimer testing as a pivotal diagnostic tool for thrombotic and cardiovascular disorders is anticipated to drive substantial growth within the market. As medical professionals become more aware about its significance, the demand for D-dimer testing is expected to increase.
Loading Table Of Content...
Loading Research Methodology...



