Deferasirox Market Research, 2033
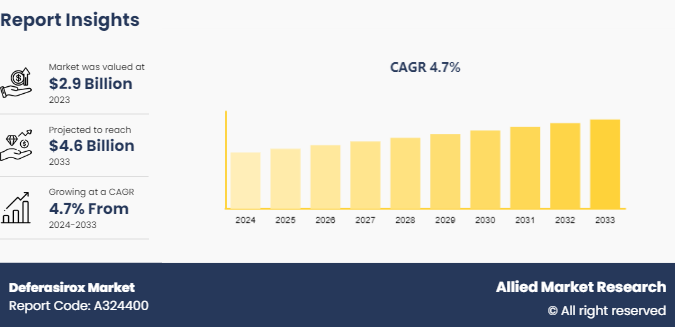
The global deferasirox market size was valued at $2.9 billion in 2023, and is projected to reach $4.6 billion by 2033, growing at a CAGR of 4.7% from 2024 to 2033. The rising prevalence of iron overload disorders such as thalassemia and sickle cell anemia, which necessitate effective iron chelation therapies, increasing awareness and diagnosis rates, along with the availability of improved healthcare infrastructure, are contributing to market growth.
Market Introduction and Definition
Deferasirox is an oral iron chelator used to treat chronic iron overload caused by blood transfusions, commonly seen in conditions such as thalassemia and sickle cell anemia. It binds to excess iron, forming a complex excreted primarily via feces. This helps prevent iron-induced damage to vital organs, including the heart and liver. Deferasirox is available under trade names such as Exjade and Jadenu and is usually administered once daily. It is generally well-tolerated but may cause side effects such as gastrointestinal disturbances, skin rash, and elevated liver enzymes. Regular monitoring is essential to manage potential toxicities and ensure effective treatment.
Key Takeaways
- The deferasirox market share study covers 20 countries. The research includes a segment analysis of each country in terms of value for the projected period.
- More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major deferasirox industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrated high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and to assist stakeholders in making educated decisions in order to achieve their most ambitious growth objectives.
Key Market Dynamics
The rising prevalence of blood disorders such as thalassemia, sickle cell anemia, and myelodysplastic syndromes necessitates frequent blood transfusions, leading to chronic iron overload, which requires effective chelation therapy. This drives the incidence of transfusion-dependent anemias, which directly fuels the demand for deferasirox. In addition, advancements in diagnostic techniques have improved the early detection and diagnosis of these conditions, thereby increasing the number of patients eligible for deferasirox therapy. Furthermore, the drug's oral administration route offers a significant advantage over older chelation therapies that required subcutaneous infusions, improving patient compliance and quality of life, which further supports the deferasirox market growth.
Moreover, the pharmaceutical companies are investing in research and development to enhance the efficacy and safety profile of deferasirox, leading to new formulations and combination therapies that broaden its application and reduce side effects thereby drives the market growth. Furthermore, patient awareness campaigns and education initiatives are increasing the understanding of the risks associated with iron overload and the benefits of chelation therapy, driving higher treatment rates. The growing geriatric population, which is more susceptible to chronic conditions requiring transfusions, also contributes towards the expansion during deferasirox market forecast period.
Economic factors such as rising disposable incomes, especially in developing countries, enable more patients to afford advanced treatments. The expanding health insurance coverage globally, including for chronic diseases and associated treatments, reduces the financial burden on patients, facilitating greater adoption of deferasirox. In parallel, government policies and funding aimed at improving healthcare outcomes contribute significantly to deferasirox market size. The competitive landscape is marked by the presence of key players investing in marketing strategies to increase product visibility and market share.
However, the potential side effects such as kidney and liver issues may deter usage, and the availability of alternative iron chelating agents might reduce market share. In addition, regulatory challenges and stringent approval processes can also delay market entry and expansion. On the other hand, expanding healthcare infrastructure and rising healthcare expenditure in emerging markets provides a deferasirox market opportunity.
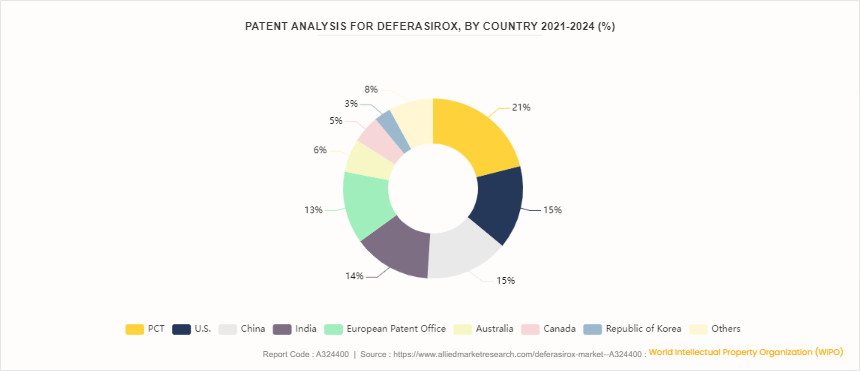
Patent Analysis for Deferasirox, By Country 2021-2024
According to World Intellectual Property Organization (WIPO), from 2021 to 2024, patent activity for Deferasirox reveals significant global interest, with key regions demonstrating varying levels of innovation. The Patent Cooperation Treaty (PCT) holds the largest share at 21%, indicating a strong international filing trend for this iron-chelating agent. The U.S. and China each contribute 15% to the total patent filings, reflecting robust research and development efforts in these major pharmaceutical markets. India follows closely with 14%, showcasing a growing focus on Deferasirox within the Indian pharmaceutical sector. The European Patent Office (EPO) accounts for 13% of the patents, emphasize the importance of Deferasirox in Europe. Australia and Canada contribute 6% and 5%, respectively, while South Korea's share stands at 3%. The others category, at 8%, includes various other countries engaged in developing Deferasirox-related technologies. This patent distribution highlights global strategic interest in Deferasirox, paralleling the drug's expanding market presence. As the drug continues to address iron overload disorders, these patent figures suggest a competitive landscape with significant contributions from leading pharmaceutical hubs..
Market Segmentation
The deferasirox market is segmented into product type, application, distribution channel, and region. On the basis of the product type, the market is segmented into 90mg, 125 mg, 250 mg, 360 mg, and others. By application, the market is classified into transfusional iron overload and NTDT caused iron overload. By end user, the market is divided into hospital pharmacies, retail pharmacies, and online providers. By region, it is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional/Country Market Outlook
North America represents a significant portion of the deferasirox market share, owing to its advanced healthcare infrastructure, high prevalence of congenital hemolytic anemias, and substantial patient awareness. In addition, extensive research and development activities, along with strong support from healthcare policies that facilitate access to advanced treatments contributes to the market growth.
The Asia-Pacific region is emerging as a lucrative market for deferasirox, driven by rapid urbanization, expansion of healthcare access, and increase in awareness of genetic blood disorders. Countries such as China, India, and Japan are at the forefront of this growth. China and India, with their large populations, have a high incidence of congenital hemolytic anemias, necessitating effective iron chelation therapies. Moreover, initiatives by governments to improve healthcare accessibility in rural areas are expected to drive market expansion in the region.
Industry Trends
- In July 2023, the World Health Organization (WHO) published the Model Lists of Essential Medicines (EML) and Essential Medicines for Children (EMLc) , including for the first time ever all three iron chelating drugs used to treat patients with thalassemia; deferoxamine (DFO) , deferiprone (DFP) , and deferasirox (DFX) . This endorsement boosts the credibility and adoption of deferasirox in treating thalassemia, potentially increasing the market growth
Competitive Landscape
The major players operating in the deferasirox market include Novartis AG, Zydus Group, Dr. Reddy’s Laboratories, Inc., Teva Pharmaceutical Industries Ltd., Sun Pharmaceutical Industries Ltd., MSN Laboratories, Glenmark Pharmaceuticals Inc., ?NATCO Pharma Limited, Taj Pharmaceuticals Limited, and ?Viatris Inc. Other players in the deferasirox market include Camber Pharmaceuticals, Inc. and so on.
Recent Key Strategies and Developments in Deferasirox Industry
- In August 2023, Camber Pharmaceuticals announced the addition of Deferasirox Oral Granules to its current portfolio. Deferasirox oral granules are indicated for the treatment of chronic iron overload (1) due to blood transfusions (transfusional hemosiderosis) in patients 2 years of age and older, and (2) in patients 10 years of age and older with non-transfusion-dependent thalassemia (NTDT) syndromes exhibiting elevated liver and serum iron concentrations.
Key Sources Referred
- National Center for Biotechnology and Information (NCBI)
- Centers for Medicare & Medicaid Services (CMS)
- National Health Service (NHS)
- Australian Government Department of Health and Aged Care
- Government of Canada's Health and Wellness
- Ministry of Health and Family Welfare (MoHFW)
- National Health Mission (NHM)
- Ayushman Bharat - Health and Wellness Centers (AB-HWCs)
- Centers for Disease Control and Prevention (CDC)
- Food and Drug Administration (FDA)
- National Institutes of Health (NIH)
- World Health Organization (WHO)
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the deferasirox market analysis from 2024 to 2033 to identify the prevailing deferasirox market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the deferasirox market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global deferasirox market trends, key players, market segments, application areas, and market growth strategies.
Deferasirox Market , by Product Type Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 4.6 Billion |
| Growth Rate | CAGR of 4.7% |
| Forecast period | 2024 - 2033 |
| Report Pages | 230 |
| By Product Type |
|
| By Application |
|
| By Distribution Channel |
|
| By Region |
|
| Key Market Players | Glenmark Pharmaceuticals Inc., Teva Pharmaceutical Industries Ltd., Zydus Group, Dr. Reddy’s Laboratories, Inc., Novartis AG, Taj Pharmaceuticals Limited, MSN Laboratories , Viatris Inc., Sun Pharmaceutical Industries Ltd., NATCO Pharma Limited |
The total market value of deferasirox market is $2.9 billion in 2023.
The market value of deferasirox market in 2033 is $4.6 billion.
The base year is 2023 in deferasirox market.
The forecast period for deferasirox market is 2024 to 2033.
North America has significant share in 2023, owing to well established healthcare infrastructure, substantial investments in research and development, and the widespread availability of deferasirox products.
The key drivers include the increasing prevalence of iron overload disorders, improved diagnosis and awareness, advancements in pharmaceutical formulations, expanded patient access programs, and supportive government initiatives.
Deferasirox is an oral iron chelation therapy used to treat chronic iron overload due to blood transfusions in conditions such as thalassemia and sickle cell anemia.
Loading Table Of Content...



