Dry Eye Syndrome Treatment Market Research, 2032
The global dry eye syndrome treatment market was valued at $4.7 billion in 2022, and is projected to reach $9.3 billion by 2032, growing at a CAGR of 7.1% from 2023 to 2032. A dry eye syndrome occurs when the quantity and/or quality of tears fails to keep the surface of the eye properly lubricated. The disease causes a scratchy sensation or a feeling that something is in the eye. Other symptoms include stinging or burning, episodes of excess watering following periods of stress, discharge, pain, and redness in the eye. It leads to increased ocular surface inflammation and damage

Market Dynamics
The increase in prevalence of eye diseases such as dry eyes, eye inflammation, and cataract boost the growth of the dry eye syndrome treatments industry. For instance, according to according to investor presentation of Bausch + Lomb, a leading eye care company, in U.S. an estimated 38 million people affected with dry eye disease (DED) out of which only 19 million were diagnosed. Thus, the growing prevalence of dry eyes syndrome (DES) drives the Dry Eye Syndrome Treatment Market Growth.
Moreover, the rise in the geriatric population and increase in screen time are expected to create remunerative opportunities for the expansion of the market. Geriatric people commonly have eye related problems such as cataracts, vision impairment and dry eye syndrome. For instance, according to the report published by the America’s Health Ranking by United Health Foundation, in 2021, it has been estimated that more than 54 million adults ages 65 and older live in the U.S. that is about 16.5% of the nation’s population.
In addition, key players have adopted various strategies such as product launches, acquisitions, agreements, partnerships, and collaborations to strengthen their foothold in the Dry Eye Syndrome Treatment Industry. For instance, in May 2023, Bausch + Lomb Corporation, a leading global eye health company, and Novaliq GmbH, a biopharmaceutical company focusing on first- and best-in class ocular therapeutics, announced that the U.S. Food and Drug Administration (FDA) has approved MIEBO (perfluorohexyloctane ophthalmic solution; formerly known as NOV03), for the treatment of the signs and symptoms of dry eye disease (DED). MIEBO is the first and only FDA-approved treatment for DED that directly targets tear evaporation.
The demand for dry eye syndrome treatments is not only limited to developed countries but is also being witnessed in developing countries, such as China, Brazil, and India, which fuels the growth of the Dry Eye Syndrome Treatment Market Size. Factors such as a rise in hospital admissions, an increase in the number of surgeries, and a surge in healthcare expenditures drive the growth of the market. In addition, an increase in the use of electronic devices such as television and smartphones are expected to fuel the growth of the Dry Eye Syndrome Treatment Industry.
However, the lack of awareness about dry eye syndrome (DES) is expected to hamper market growth.
Segmental Overview
The dry eye syndrome treatment market is segmented into drug, dosage form, distribution channel, and region. On the basis of drugs, the market is classified into lubricants, dry eye syndrome drug, and others. On the basis of dosage form, the market is classified into eye drops, ointment, and others. On the basis of distribution channel, it is classified into hospital pharmacies, retail pharmacies and online providers. On the basis of region, the market is studied across North America (the U.S., Canada, and Mexico), Europe (Germany, France, the UK, Italy, Spain, and rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and rest of LAMEA).
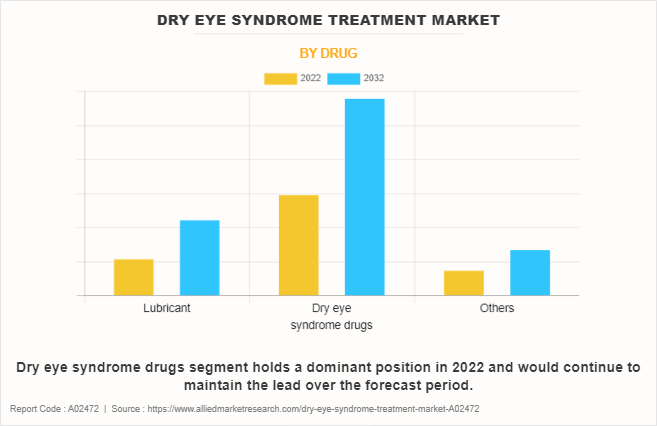
By Drug
On the basis of the drug, the market is classified into lubricant, dry eye syndrome drugs, and others. The dry eye syndrome drugs segment dominated the global Dry Eye Syndrome Treatment Market Share in 2022 and is expected to remain dominant throughout the Dry Eye Syndrome Treatment Market Forecast period owing to an increase in the prevalence of dry eye syndrome. Moreover, the surge in the geriatric population is anticipated to boost the demand for dry eye syndrome treatment. This is attributed to the fact that aged individuals are highly susceptible to eye diseases.
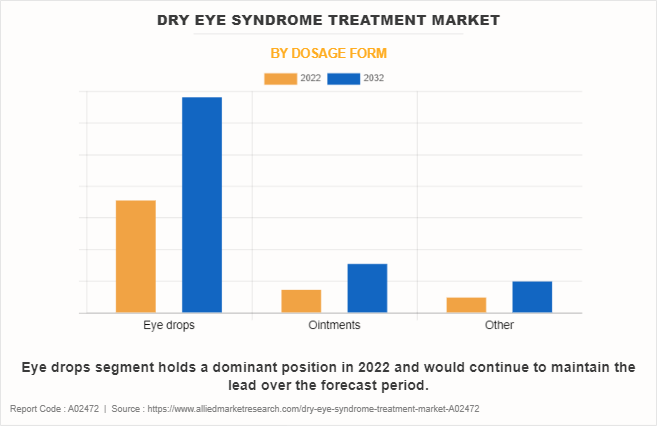
By Dosage Form
On the basis of dosage form, the market is classified into eye drops, ointments, and others. The eye drops segment dominated the global Dry Eye Syndrome Treatment Market Size in 2022 and is expected to remain dominant throughout the forecast period owing to the advantages of eye drops and the rise in the number of patients with dry eyes.
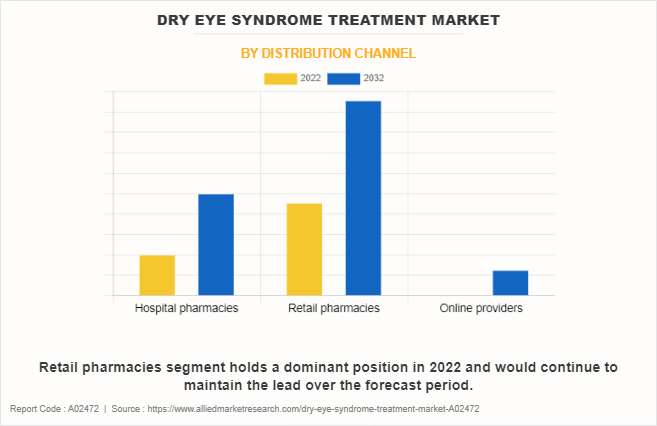
By Distribution Channel
On the basis of distribution channel, the market is divided into hospital pharmacies, retail pharmacies, and online providers. The retail pharmacies segment dominated the global Dry Eye Syndrome Treatment Market Share in 2022 and is anticipated to continue this trend during the forecast period. This is attributed to an increase in the availability of dry eye syndrome treatments at retail pharmacies. In addition, an increase in the number of retail pharmacies drives the growth of the dry eye syndrome treatment market. Moreover, improvements in the healthcare infrastructure are expected to propel the growth of the market.
By Region
On the basis of region, the dry eye syndrome treatment market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. In 2022, North America was the dominant region and is expected to continue this trend throughout the forecast period owing to high prevalence of eye disorders such as dry eyes and visual disturbances, the strong presence of key players, and the surge in the number of critically ill patients in the region. However, Asia-Pacific is expected to witness the highest CAGR during the analysis period, owing to the presence of high population countries such as India and China, which, in turn, increases the prevalence of cardiovascular disorders and the growing number of strategies and trends adopted by the market players that include product development, product approval, partnership, collaboration, and agreement.
COMPETITION ANALYSIS
Competitive analysis and profiles of the major players in the dry eye syndrome treatment market such as include Abbvie, Viatris, Novartis AG, Bausch & Lomb Incorporated, Sun Pharma, Novaliq, Santen Pharmaceutical Co., AFT Pharmaceuticals, Alcon and Johnson & Johnson. are provided in this report. These players have adopted product launch and product development as key developmental strategies to improve the product portfolio of the dry eye syndrome treatment.
Recent Acquisition in the Dry Eye Syndrome Treatment Market
In June 2023, Bausch + Lomb Corporation announced it has entered into a definitive agreement with Novartis under which Bausch + Lomb will acquire XIIDRA (lifitegrast ophthalmic solution) 5%, a non-steroid eye drop specifically approved to treat the signs and symptoms of dry eye disease (DED) focusing on inflammation associated with dry eye.
In February 2022, AbbVie, announced that it has completed its acquisition of Allergan plc following receipt of regulatory approval from all government authorities required by the transaction agreement and approval by the Irish High Court.
Recent Product Approvalin the Dry Eye Syndrome Treatment Market
In June 2023, Novaliq GmbH announced that the U.S. Food and Drug Administration (FDA) has approved VEVYE (cyclosporine ophthalmic solution) 0.1% for the treatment of the signs and symptoms of dry eye disease.
In May 2023, Bausch + Lomb Corporation and Novaliq GmbH announced that the U.S. Food and Drug Administration (FDA) has approved MIEBO (perfluorohexyloctane ophthalmic solution; formerly known as NOV03), for the treatment of the signs and symptoms of dry eye disease (DED). MIEBO is the first and only FDA-approved treatment for DED that directly targets tear evaporation.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the dry eye syndrome treatment market analysis from 2022 to 2032 to identify the prevailing Dry Eye Syndrome Treatment Market Opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the dry eye syndrome treatment market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global dry eye syndrome treatment market trends, key players, market segments, application areas, and market growth strategies.
Dry Eye Syndrome Treatment Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 9.3 billion |
| Growth Rate | CAGR of 7.1% |
| Forecast period | 2022 - 2032 |
| Report Pages | 235 |
| By Drug |
|
| By Dosage form |
|
| By Distribution Channel |
|
| By Region |
|
| Key Market Players | Novartis AG, Johnson & Johnson (J&J), Novaliq, Santen Pharmaceutical Co., Ltd., Bausch Health Companies Inc, Viatris Inc., Sun Pharmaceutical Industries Ltd., AFT Pharmaceuticals Limited, Alcon, AbbVie Inc. |
Analyst Review
Dry eye syndrome (DES) is a common condition that occurs when a patient's tears are unable to provide sufficient lubrication for their eyes. Dry eyes can be caused by many reasons such as extensive screen time, aging, and nerve damage. Dry eyes lead to discomfort, inflammation and potential damage of the eye's surface. The increase in the prevalence of dry eye syndrome, and a rise in the geriatric population drives the market growth.
In addition, the strategies such as product approvals, product launch, acquisition and merger, adopted by key players contribute to the growth of the market. For instance, in May 2023, Bausch + Lomb Corporation, a leading global eye health company, and Novaliq GmbH, a biopharmaceutical company focusing on first- and best-in class ocular therapeutics, announced that the U.S. Food and Drug Administration (FDA) has approved MIEBO (perfluorohexyloctane ophthalmic solution; formerly known as NOV03), for the treatment of the signs and symptoms of dry eye disease (DED). MIEBO is the first and only FDA-approved treatment for DED that directly targets tear evaporation.
The total market value of the Dry Eye Syndrome Treatment Market is $4725 million in 2022.
The forecast period in the report is from 2023 to 2032
North America is the largest regional market for Dry Eye Syndrome Treatment
There are 10 Dry Eye Syndrome Treatment manufacturing companies are profiled in the report.
The top companies that hold the market share in Abbvie, Viatris, Novartis AG, Bausch & Lomb Incorporated, Sun Pharma, and Novaliq
The base year for the report is 2022.
Yes, Dry Eye Syndrome Treatment companies are profiled in the report.
Yes, the competitive landscape included in the Dry Eye Syndrome Treatment market report
Loading Table Of Content...
Loading Research Methodology...



