eClinical Solutions Market Research, 2031
The global eClinical solutions market was valued at $6.82 billion in 2021, and is projected to reach $21.50 billion by 2031, growing at a CAGR of 12.2% from 2022 to 2031. Various eClinical solutions, such as electronic data capture & clinical data management systems, clinical trial management system, randomization and trial supply management, and others are used for the efficient management of data for clinical trials. It helps in effective management and integration of data generated during clinical trials. It offers set of tools to effectively plan, manage, track clinical study portfolios, and create insights. It integrates contact management sites & teams, calendar & monitoring, and document management subsequently engender acceptable clinical research outcomes and compliant submissions; data entry storage and regulation; authentication of data integrity and reliability; enabling better patient experiences by narrowing the drug development time.

The drivers contributing to the growth of the eClinical solutions market include, increase in disease load leading to drug development. eClinical solutions accelerate the decision-making process by improving data visibility. It solves data fragmentation and decision-making issues by simplification of operational data management. Technology is supposed to create new opportunities in clinical trials by solving problems regarding data management with automation of drive and the data driving process becomes easier by using eClinical solutions, thus driving eClinical solutions market growth.
Furthermore, the pace of technological advancement creates a lucrative eClinical solutions market opportunity. Continuous advancement of technologies ease data management. Newly launched eClinical solutions reduce time, enhance decision making, and help integrate multiple applications. Increased opportunities in emerging market with improved research infrastructure, and development in need of personalized medicines is expected to drive the development throughout the forecast period. High initial capital investment and maintenance cost of eClinical solutions are projected to restrain market expansion, particularly poor reimbursement circumstances.
Impact of COVID-19
The COVID-19 outbreak is anticipated to have a positive impact on the eClinical solutions market. A huge number of clinics and hospitals across the globe were restructured to increase hospital capacity for patients diagnosed with COVID-19. Non-essential procedures took a potential backlog, owing to rapidly rising COVID-19 cases. The lockdown led to disruption of manufacturing and transportation of healthcare essentials. Furthermore, other factors responsible for the impact on the market include, limited availability of medical care, shortage of healthcare staff, and rise in burden of COVID-19 related hospitalization.
Vast data scale from the healthcare industry, increase in the number of clinical trials, and government emphasis on clinical research after the pandemic drive the market growth. Thus, the COVID-19 outbreak is anticipated to have a positive impact on the growth of the global eClinical solutions market forecast.
eClinical Solutions Market Segmentation
The global eClinical solutions market is segmented on the basis of product, delivery mode, clinical trials, end user, and region. By product, it is divided into electronic data capture (EDC) & clinical data management systems (CDMS), clinical trial management systems (CTMS), randomization and trial supply management (RTSM), electronic clinical outcome assessment (eCOA), electronic trial master file (eTMF), and others. By delivery mode, it is divided into web-hosted, on-premise, and cloud-based. By clinical trials, it is segmented into phase I, phase II, phase III, and phase IV. By end user, it is segmented into pharmaceutical and biopharmaceutical companies, contract research organizations, and others
Region-wise, the market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, UK, Italy, Spain, and rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and rest of LAMEA).
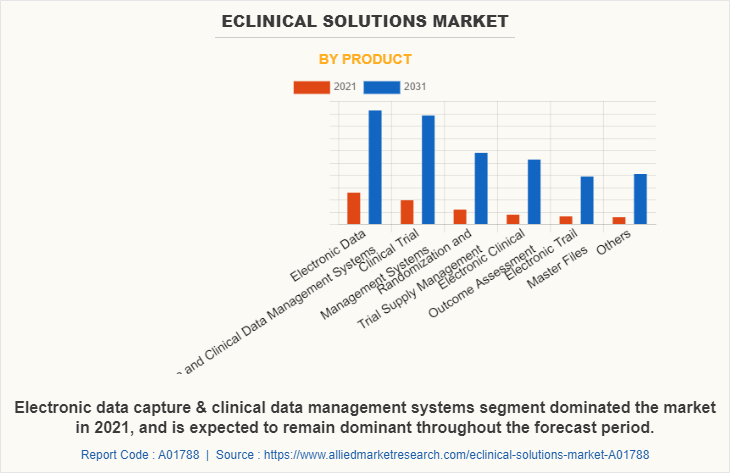
By Product
Depending on product, the electronic data capture (EDC) & clinical data management systems (CDMS) segment dominated the market in 2021, with $1789.1 million eClinical solutions market share owing to the quality of information captured, streamlines data collection procedures, and offers effective data analysis. The electronic clinical outcome assessment segment is expected to witness highest CAGR of 13.4% during the forecast period owing to rise in significance of high-quality clinical data.
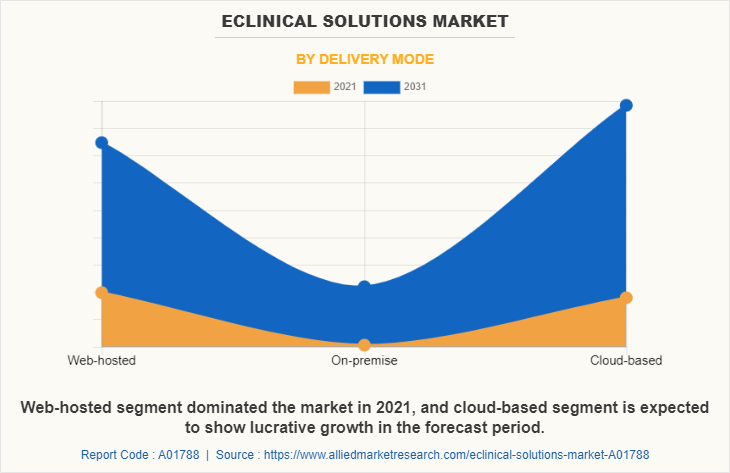
By Delivery Mode
Depending on delivery mode, the web-hosted segment dominated the market in 2021, with $2973.24 million eClinical solutions market share owing to these products having a greater level of interoperability. The cloud-based segment is expected to witness highest CAGR of 13.4% during the eclinical solutions market forecast period owing to flexibility, high accessibility, negligible handling costs, and easy data backup.
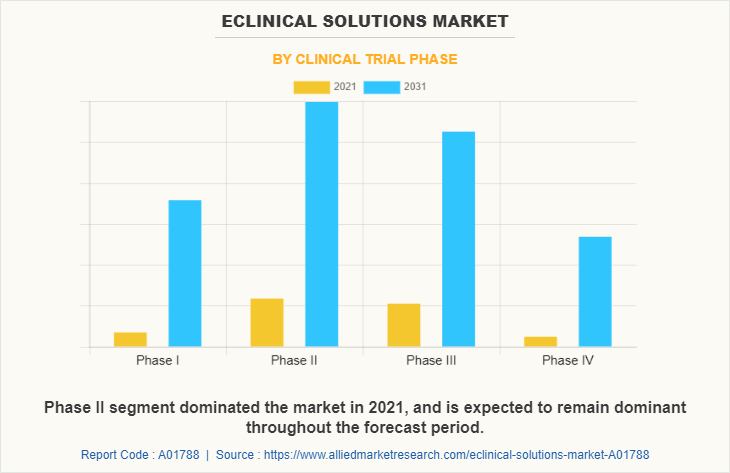
By Clinical Trials Phase
Depending on clinical trials, the phase II segment dominated the eClinical solutions market size in 2021, with $2178.6 million owing to increasing number of drugs successfully reaching phase III. The phase I segment is expected to witness highest CAGR of 13.0% during the forecast period owing to high significance of these systems to predict future outcomes and eliminate drug candidates possessing the least probability of success.
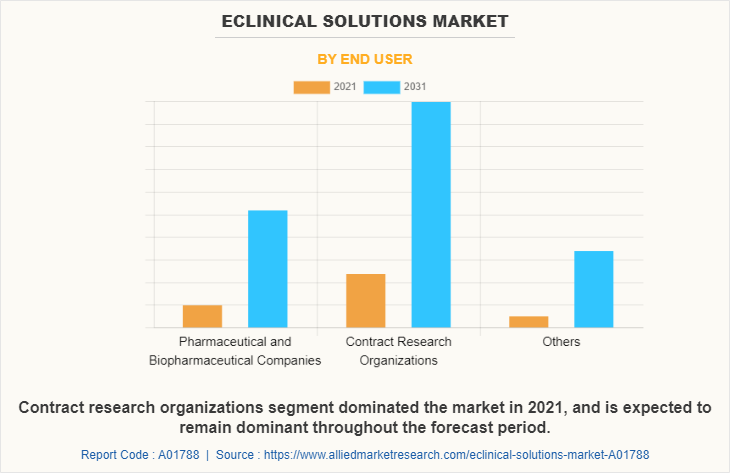
By End User
Depending on end user, the contract research organization segment dominated the eClinical solutions market size and is expected to witness highest CAGR of 12.6% during the forecast period owing to inclination of pharmaceutical companies to reduce overall expenditure improved clinical trials and streamlined research workload.
By Region
North America is expected to account for the highest market share, owing to established robust infrastructure of companies, advanced technology and availability of key players across the region, followed by Europe, Asia-Pacific, and LAMEA. Asia-Pacific is expected to register the fastest CAGR during the forecast period, due to increase in rapid development in healthcare technology, and increase in public–private investment for eClinical solutions industry.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the eClinical solutions market analysis from 2021 to 2031 to identify the prevailing eCinical solutions market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the eClinical solutions market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global eClinical solutions market trends, key players, market segments, application areas, and market growth strategies.
eClinical solutions Market Report Highlights
| Aspects | Details |
| By Delivery Mode |
|
| By Clinical Trial Phase |
|
| By Product |
|
| By End User |
|
| By Region |
|
| Key Market Players | Dassault Systemes (Medidata Solution, Inc.), DataTrak International, Inc., eClinical Solutions, Inc., Castor EDC, MaxisIT Inc., Signant Health (CRF Health Inc.), Parexel International Corporation (Calyx), Anju Software, Inc., IBM Watson Health, Oracle Corporation, Advarra, Inc. (Bio Optronics, Inc.), MedNet Solutions, Medrio, Inc., Clario, Business Systems Integration AG, Saama Technologies, Inc., veeva systems |
Analyst Review
Development of advanced and reliable eClinical solutions systems has led to rise in the number of applications of eClinical solutions. Increase in demand for data integration systems and better healthcare services are also expected to propel the growth of the eClinical solutions market.
The eClinical solutions industry holds the high potential, owing to innovative concepts and multidisciplinary expertise demand for new technological advancements in the creation of eClinical solutions. The recommendations for them are anticipated to expand with the introduction of new software with high accuracy and integration between various softwares. Increase in use of eClinical solutions techniques by researchers have led to an enhancement of these software capable of exponential growth of market
The forecast period of eClinical solutions market is 2022 to 2031.
The base year for eClinical solutions market is 2021.
The global eClinical solutions market was valued at $6,816.17 million in 2021, and is estimated to reach $21,502.95 million by 2031, growing at a CAGR of 12.2% from 2022 to 2031.
Oracle Corporation, Parexel International Corporation (Calyx), DataTrak International, Inc., Dassault Systèmes (Medidata Solution, Inc.), Clario, Signant Health (CRF Health Inc.),IBM Watson Health,Veeva Systems are the top companies to hold the market share in eClinical solutions market.
Electronic data capture (EDC) & clinical data management systems (CDMS) is the leading product type of eClinical solutions market.
Surge in the number of drug development studies, and rise in the number of clinical trials are the upcoming trends of eClinical solutions market.
North America is the largest regional market for eClinical solutions market.
Loading Table Of Content...


