Helicobacter Pylori Diagnostics Market Research, 2032
The global helicobacter pylori diagnostics market size was valued at $552.7 million in 2022 and is projected to reach $926.8 million by 2032, growing at a CAGR of 5.3% from 2023 to 2032. Helicobacter pylori (H. pylori) is a bacterium that infects the stomach and is associated with various gastrointestinal disorders, such as peptic ulcer disease and gastritis.
There are several methods for diagnosing H. pylori infection such as breath test, stool antigen test, and rapid urease test. H. pylori (Helicobacter pylori) is a type of bacteria that can infect the stomach and the small intestine. It is a common bacterial infection, with an estimated two-thirds of the world's population being infected. H. pylori causes various gastrointestinal problems, including gastritis (inflammation of the stomach lining), peptic ulcers (sores in the stomach or duodenum), and even stomach cancer in rare cases. Common symptoms of H. pylori infection include abdominal pain, nausea, vomiting, loss of appetite, and weight loss.
Market Dynamics
H. pylori infection is a major risk factor for gastric cancer. Increase in the incidence of gastric cancer there is a growing demand for early detection and treatment of H. pylori infections that propels helicobacter pylori diagnostics market growth. For instance, American Cancer Society’s estimated for stomach cancer (also known as gastric cancer) in the U.S. 2023. Thus, the rise in cases of gastric cancer drives the demand for H. pylori diagnostic testing and further propels market growth.
In addition, growing demand for non-invasive tests boosts market growth. Patients prefer non-invasive diagnostic tests such as breath tests and stool antigen tests over invasive tests such as endoscopy. Thus, demand for non-invasive tests has driven the development of new diagnostic technologies which further fuel the growth during helicobacter pylori diagnostics market forecast.
The growth of the helicobacter pylori diagnostics market is expected to be driven by high potential in untapped, emerging markets, due to availability of improved healthcare infrastructure, increase in unmet healthcare needs, and rise in prevalence of infectious diseases.
Furthermore, the healthcare industry in emerging economies is developing at a significant rate, owing to rise in demand for enhanced healthcare services, significant investments by government to improve healthcare infrastructure, and development of the medical tourism industry in emerging countries.
Moreover, early diagnosis and treatment of H. pylori infections can help to prevent the development of more serious complications, such as gastric cancer that supports market growth. As a result, there is an increase in demand for H. pylori testing to facilitate early detection and treatment. H. pylori infections are associated with several gastrointestinal diseases, including peptic ulcer disease and gastric cancer. Thus, a rise in awareness of these diseases led to an increase in the demand for H. pylori testing and further boost the grwoth of the helicobacter pylori diagnostics market size.
The demand for helicobacter pylori diagnostics is not only limited to developed countries but is also being witnessed in the developing countries, such as China, Brazil, and India, which fuel the growth of the market. Factors such as rise in adoption of point of care testing, and increase in awareness toward early diagnosis, further drive the growth of the market.
Lack of awareness about the H. pylori infection among the general population is a restraining factor for the growth of the H. pylori diagnostics market. Many people are not aware of the symptoms and complications associated with H. pylori infection, which leads to delayed diagnosis and treatment. Regulatory issues related to the approval of new diagnostic tests for H. pylori may also act as a restraining factor for market growth. The regulatory approval process is time-consuming and expensive, which may limit the availability of new and innovative H. pylori diagnostics tests in the market.
The outbreak of COVID-19 has disrupted workflows in the health care sector around the world. The disease has forced a number of industries to shut their doors temporarily, including several sub-domains of health care. The global helicobacter pylori diagnostics industry experienced a decline in 2021 due to global economic recession led by COVID-19. The COVID-19 pandemic has led to the postponement or cancellation of non-urgent diagnostic procedures, including H. pylori testing. This has resulted in a decline in the demand for H. pylori testing, which has affected the revenue of companies operating in this market. In addition, pandemic also led to a decrease in patient visits to healthcare facilities due to fear of infection and the implementation of social distancing measures. This reduced the number of patients undergoing H. pylori testing, which impacted the market negatively.
However, the market is recovering after 2022, and show stable growth for the Helicobacter pylori diagnostics market in the coming future. This is attributed to the development of innovative and cost-effective diagnostic technologies and the availability of effective treatment options for H. pylori infection are expected to drive the growth of the H. pylori diagnostics market in the coming years.
Segmental Overview
The helicobacter pylori diagnostics market is segmented into test type, method, end user, and region. By test type, the market is categorized into non-invasive test and invasive test. The non-invasive test segment is further divided into the serology test, stool antigen test, and carbon urea breath test. On the basis of method, the market is segregated into laboratory-based test and point of care test. By end user, the market is classified into hospitals, diagnostic laboratories, and others. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
By Test Type:
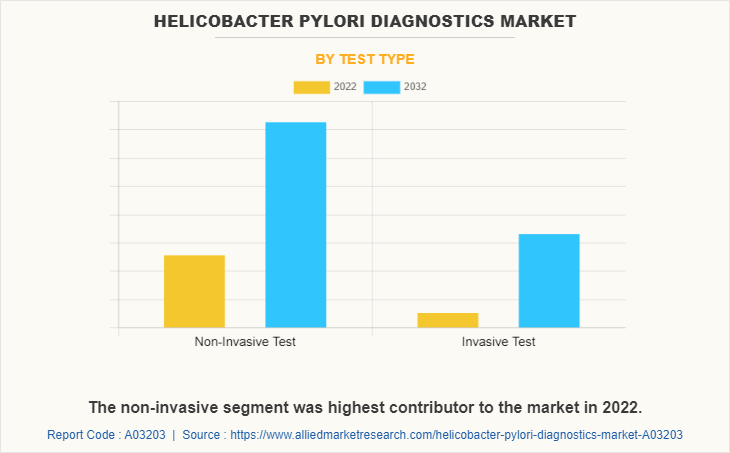
The non-invasive tests segment dominated the global helicobacter pylori diagnostics market share in 2022 and is expected to remain dominant throughout the forecast period, owing to several advantages over invasive tests, the development of advanced non-invasive testing technologies, and the increase in cases of gastric ulcers associated with H. pylori gastritis.
By Method:
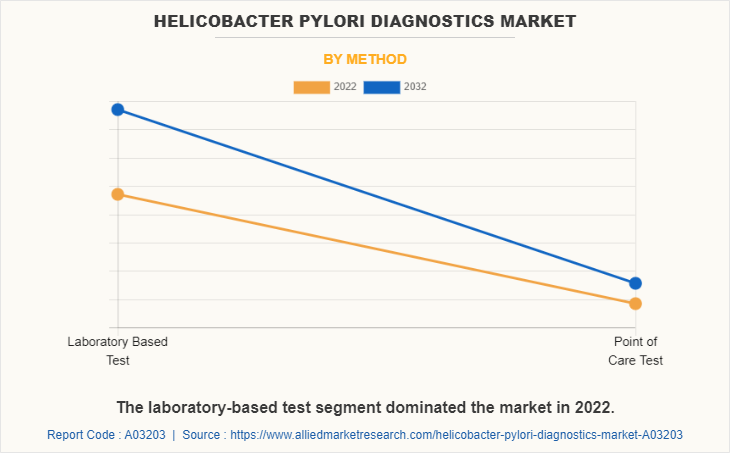
The laboratory-based tests segment dominated the global market in 2022 owing to high adoption of laboratory-based tests such as immunoassay due to their accuracy, easy to use, and easy availability. However, the point of care (POC) test segment is expected to register the highest CAGR during the forecast period owing to its easy accessibility, high portability, and increasing adoption by healthcare providers.
By End User:
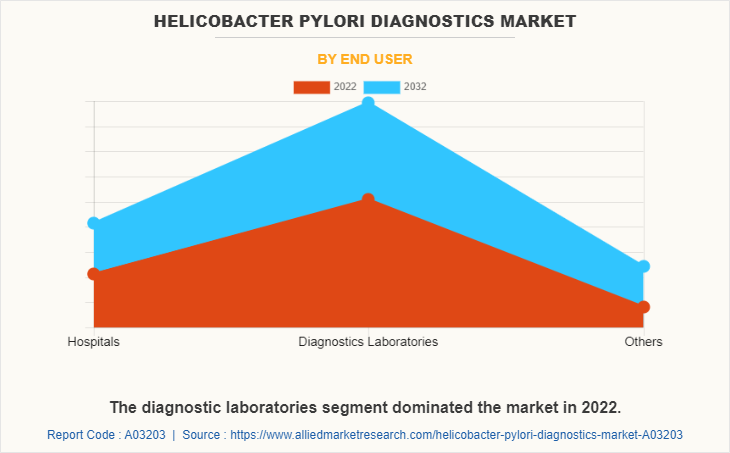
The diagnostic laboratories segment held the largest market share in 2022 and is expected to remain dominant throughout the forecast period, owing to the availability of advanced laboratory facilities, the expertise of laboratory professionals, regulatory compliance, range of testing services, and easy accessibility. In addition, diagnostic laboratories offer a wide range of testing services, including non-invasive and invasive tests, which allows healthcare providers to choose the most appropriate test for each individual patient that supports the market growth.
By Region:
The Helicobacter pylori diagnostics market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for a major helicobacter pylori diagnostics market share in 2022 and is expected to maintain its dominance during the forecast period.
Increase in the incidence of gastric diseases such as H. pylori infection is a risk factor for gastric diseases that fuels the demand for H. pylori diagnostic tests. In addition, early detection of H pylori infection is crucial for effective management and prevention of associated complications which drives the demand for H pylori diagnostics tests and propels the market growth in this region. In addition, rise in awareness among patients and healthcare providers about the diagnosis of H. pylori infection leading to an increase in demand for H. pylori tests and further support the market growth.
Furthermore, North America has the highest healthcare spending which enables better access to diagnosis tests for H pylori testing is expected to contribute towards the market growth. Furthermore, the presence of well-established healthcare infrastructure, high purchasing power, and rise in adoption rate of helicobacter pylori diagnostics tests are expected to drive the market growth. Moreover, product approvals, acquisitions, and agreement adopted by the key players in this region boost the growth of the market.
Asia-Pacific is expected to grow at the highest rate during the forecast period. The market growth in this region is attributable to high incidences of gastric ulcer fuels the demand for H pylori diagnostic tests as well as growth in the purchasing power of populated countries, such as China and India. In addition, significant advancements in diagnostic technologies for H. pylori infection such as urea breath test drive the market growth. These technologies offer convenient and reliable alternatives to invasive methods which increase their adoption and drive the growth of the helicobacter pylori diagnostics industry.
Competition Analysis
Competitive analysis and profiles of the major players in the helicobacter pylori diagnostics, such as Abbott Laboratories, Avanos Medical, Inc., Biohit Oyj, Bio-Rad Laboratories, Inc., Cardinal Health Inc., F. Hoffmann-La Roche Ltd., Gulf Coast Scientific, Meridian Bioscience, Inc., Quidel Corporation, and Thermo Fisher Scientific Inc. are provided in this report. Major players have adopted product approval, agreement and acquisition as key developmental strategies to improve the product portfolio of the helicobacter pylori diagnostics market.
Recent Product Approval in the Helicobacter Pylori Diagnostics Market
- In March 2020, Meridian Bioscience, Inc. announced that it received FDA-clearance for Meridian’s new Curian and Curian HpSA assay.
- In August 2021, the latest innovation in the unique Biohit GastroPanel product family, GastroPanel quick test, received the CE marked approval.
Acquisition in the Helicobacter Pylori Diagnostics Market
- In January 2023, Meridian Bioscience, Inc. and SD Biosensor, Inc. and SJL Partners LLC announced that they have completed the transaction to acquire Meridian.
Agreement in the Helicobacter Pylori Diagnostics Market
- In July 2021, Meridian Bioscience, Inc., a leading global provider of diagnostic testing solutions and life science raw materials, announced that it signed a definitive agreement to acquire the North American BreathTek business from Otsuka America Pharmaceutical, Inc.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the helicobacter pylori diagnostics market analysis from 2022 to 2032 to identify the prevailing helicobacter pylori diagnostics market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the helicobacter pylori diagnostics market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global helicobacter pylori diagnostics market trends, key players, market segments, application areas, and market growth strategies.
Helicobacter Pylori Diagnostics Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 926.8 million |
| Growth Rate | CAGR of 5.3% |
| Forecast period | 2022 - 2032 |
| Report Pages | 261 |
| By Test Type |
|
| By Method |
|
| By End User |
|
| By Region |
|
| Key Market Players | F. Hoffmann-La Roche Ltd., Meridian Bioscience, Inc., Bio-Rad Laboratories, Inc. , Quidel Corporation, Cardinal Health Inc., Gulf Coast Scientific, Thermo Fisher Scientific Inc. , Abbott Laboratories, Biohit Oyj, Avanos Medical, Inc. |
Increase in awareness about the importance of early diagnosis of H. pylori infection, rise in advancements in Helicobacter pylori diagnostics led to the development of more accurate and reliable tools, and surge in demand for minimally invasive methods for H. pylori testing are the factors responsible for the market growth.
The non-invasive test segment is the most influencing segment owing to high adoption of non-invasive tests such as serology and stool antigen tests as they are easy to perform and requires only a blood or stool sample.
Helicobacter pylori diagnostics refer to the various methods and tests used to detect the presence of H. pylori bacteria or associated antibodies in individuals of having H. pylori infection.
Top companies such as Abbott Laboratories, Thermo Fisher Scientific Inc., F. Hoffamann-La-Roche Ltd., and Bio-Rad Laboratories Inc. held high market share in 2022.
The base year is 2022 in Helicobacter pylori diagnostics market.
North America is the largest regional market for Helicobacter pylori diagnostics owing to easy availability of products for diagnosis of H. pylori bacteria, advanced healthcare infrastructure, rise in adoption of non-invasive method, and surge in adoption of advanced tools for H. pylori testing.
The estimated industry size of Helicobacter pylori diagnostics in 2032 is $926.79 million.
The forcast period for Helicobacter pylori diagnostics market is 2023 to 2032
Loading Table Of Content...
Loading Research Methodology...



