Intrapartum Monitoring Devices Market Research, 2031
The global intrapartum monitoring devices market was valued at $826.00 million in 2021 and is estimated to reach $1,624.43 million by 2031, growing at a CAGR of 7% from 2022 to 2031. The intrapartum monitoring devices are used to record changes in the fetal heart rate (FHR) through Doppler ultrasound or direct fetal ECG measurement with a fetal scalp electrode, temporal relationship to myometrial activity and uterine contractions. The interpretation of the data collected depends on the relationship between the two traces. The significant application of the intrapartum monitoring devices is to identify babies that may be hypoxic. Hence, the additional assessments of fetal well-being may be made. Otherwise, the baby can be delivered by caesarean section or instrumental vaginal birth. The intrapartum monitoring by the external devices includes fastening of ultrasound probe (transducer) to the female belly. It sends the sounds of the baby’s heart to a computer. The rate and pattern of the baby’s heart rate are shown on a screen and printed on paper.

Historical Overview
The market has been analyzed qualitatively and quantitatively from 2021 to 2031. The intrapartum monitoring devices market witnessed growth at a CAGR of around 7% from 2022 to 2031. Most of the growth during this period was from North America owing to the increase in the adoptions of intrapartum monitoring devices, rise in incidences of preterm births among population, as well as well-established presence of domestic market players in the region.
Market Dynamics
Growth & innovations in the medical devices industry for the adoption of intrapartum monitoring technology is driven by the massive pool of birth related conditions like preterm births drives the growth of Intrapartum Monitoring Devices Market Size. The rise in the tendency of couples to conceive a child safely creates an opportunity for the Intrapartum Devices Industry. Moreover, rise in number of strategies like agreement by various key players across the globe is set to affect the growth Intrapartum Monitoring Devices Market Size positively. For instance, in April 2019, Henry Schein Medical, announced the signing of an exclusive distribution and supply agreement for the MERIDIAN M110 Fetal Monitoring System. The MERIDIAN M110, currently available to Henry Schein customers, is an intrapartum fetal monitor that externally measures and displays fetal heart rate (FHR), maternal heart rate (MHR), and uterine contractions (UA). The growth of the intrapartum monitoring devices market is also expected to be driven by high potential in adoption of the technology in developing countries due to increase in the prevalence of birth disorders, increase in practice to monitor the fetal health, and surge in demand for intrapartum monitoring devices.
Furthermore, the healthcare industry in emerging economies is developing at a significant rate, owing to rise in demand for enhanced medical technologies, significant investments by government to improve fetal health monitoring, and strong foothold of biotechnological industries in emerging countries. In addition, the cost effectiveness of the intrapartum monitoring devices may also decide the demand for adoption of the devices. It is because increased cost of the devices makes it unaffordable for the facilities or hospitals that generate low revenue for the adoption of healthcare devices. Hence, the cost effectiveness factor for the intrapartum monitoring devices is attributed to further support the market growth. The demand for intrapartum monitoring devices is not only limited to developed countries but is also being witnessed in the developing countries, such as China, Brazil, and India, which fuel the Intrapartum Monitoring Devices Market Growth.
Factors such as rise in the adoption of fetal monitoring devices for the safety measurement among couples for their child and an increase in awareness toward birth disease risks further drive the growth of the market. Furthermore, a rise in consumer awareness related to preventive healthcare of newborns boosts the adoption of intrapartum monitoring devices. Moreover, an increase in research activities by manufacturers for developing innovative solutions in intrapartum monitoring device technology is expected to fuel their adoption in the near future.
However, the stringent regulations for the approval of intrapartum monitoring devices and problems regarding the lack of access of women to intrapartum care facilities in underdeveloped countries are hampering the market growth.
The intrapartum monitoring devices market is segmented into Product Type, Method, and End User.
The intrapartum monitoring devices market is segmented on the basis of product type, method, end user, and region. On the basis of product type, the market is bifurcated into monitors and electrodes. On the basis of the method, the market is classified into invasive and non-invasive. On the basis of end user, the market is classified into hospitals, maternity centers and others. Region-wise, the market is studied across North America (the U.S., Canada, and Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea and rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and rest of LAMEA).
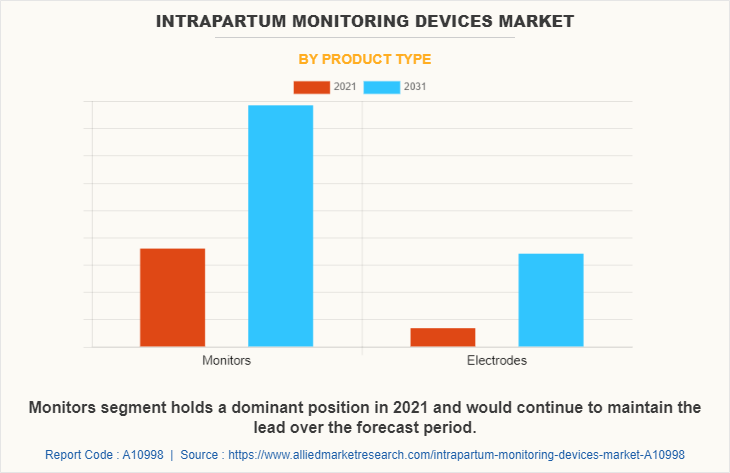
By Product Type
The monitors segment dominated the global market in 2021 and is expected to remain dominant throughout the Intrapartum Monitoring Devices Market Forecast period, owing to increase in adoption of intrapartum monitors for fetal monitoring health and availability of several types of devices that are developed by various key players in the market. Moreover, rise in the practice of at home monitoring of fetal health also boosts the demand for at home fetal health monitoring devices.
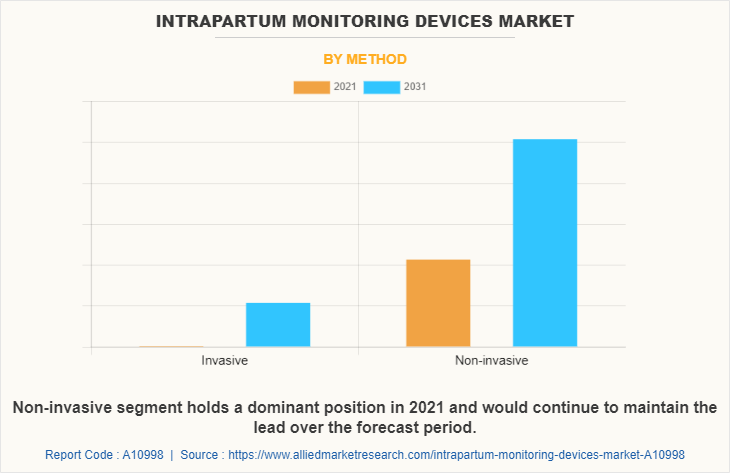
By Method
The non-invasive segment dominated the global market in 2021 and is anticipated to continue this trend during the forecast period. This is attributed to the increase in prevalence of birth disorders among the conceiving female population. In addition, growth in awareness regarding the fetal health monitoring along with mother health monitoring.
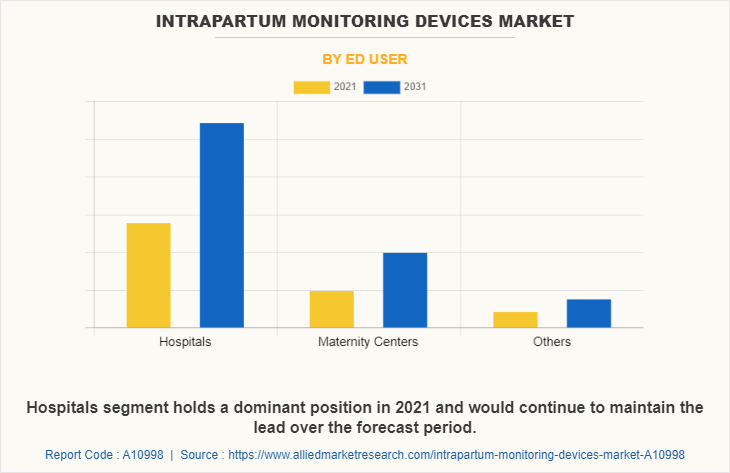
By End User
The hospitals segment held the largest Intrapartum Monitoring Devices Market Share in 2021 and is expected to remain dominant throughout the forecast period, owing to the preference of population for the intrapartum monitoring in hospitals and ease of the accessibility of the patients to the hospitals and clinics post COVID-19 pandemic situation.
By Region
The intrapartum monitoring devices market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. In 2021, North America was the dominant region and is expected to remain dominant throughout the forecast period, owing to high prevalence rate of birth disorders like preterm births, increase in the number of market players and surge in the number of devices available in the region. However, Asia-Pacific is expected to witness the highest CAGR during the analysis period, owing to the presence of high populace countries such as India and China, which in turn increases the number of chances of suffering from birth defect conditions, and the increasing number of strategies and trends adopted by the market players.
COMPETITION ANALYSIS
Competitive analysis and profiles of the major players in the intrapartum monitoring devices, such as Cardinal Health, Inc., Cooper Companies Inc., General Electric Company, Huntleigh Healthcare Limited, Koninklijke Philips N.V., Laborie, MedGyn products, Inc., Mindchild Medical, Inc., Rocket Medical plc, Stalwart Meditech are provided in this report. There are some important players in the market such as Cardinal Health, Inc., Cooper Companies Inc., General Electric Company, Koninklijke Philips N.V. Various players have adopted strategies like product launch, and acquisition as key developmental strategies to improve the product portfolio of the intrapartum monitoring devices market.
Product Launch in the market
In June 2020, Royal Philips announced an addition to its remote patient monitoring suite supporting at-risk populations during the COVID-19 emergency with the launch of a fetal health monitoring device. The new wireless Avalon CL Fetal and Maternal Pod and Patch aim to reduce unnecessary physical interactions between clinicians and patients, which is of particular importance during the COVID-19 pandemic.
Acquisitions in the market
In March 2021, Cooper Companies Inc. announced that CooperSurgical has acquired Safe Obstetric Systems, a privately held manufacturer of the medical device, Fetal Pillow. This FDA approved product is used to elevate the fetal head during a fully dilated cesarean section making the delivery easier and less traumatic for the mother and baby.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the intrapartum monitoring devices market analysis from 2021 to 2031 to identify the prevailing Intrapartum Monitoring Devices Market Opportunity
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the intrapartum monitoring devices market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global intrapartum monitoring devices market trends, key players, market segments, application areas, and market growth strategies.
Intrapartum Monitoring Devices Market Report Highlights
| Aspects | Details |
| Market Size By 2031 | USD 1.6 billion |
| Growth Rate | CAGR of 7% |
| Forecast period | 2021 - 2031 |
| Report Pages | 221 |
| By Product Type |
|
| By Method |
|
| By Ed User |
|
| By Region |
|
| Key Market Players | Cardinal Health Inc., Stalwart Meditech , Cooper Companies Inc., Rocket Medical plc, General Electric Company, Mindchild Medical, Inc., Laborie, MedGyn products, inc., Huntleigh Healthcare Limited, Koninklijke Philips N.V. |
| Other Players | Olympus Corporation |
Analyst Review
In accordance to several interviews conducted, the electronic fetal monitoring (EFM) has become the mainstream in the maternal health sector. The particular increase in the adoption of electronic fetal monitoring has given boost to non-invasive assessment via Doppler ultrasound sensor or by other medium of fetal ECG (Electrocardiogram) sensor on the fetal scalp. The intrapartum monitoring devices eliminate the discomfort of managing cables, and allows women to move around and find the most comfortable position during labor, offering them more flexible birthing options, including the time of showers and tubs.
Asia-Pacific is expected to witness the highest CAGR during the analysis period, owing to the increase in the research and development expenditure for the development of the new products that increases the adoption rate of intrapartum monitoring devices, increase in awareness about the fetal monitoring applications for increasing the adoption of technology in the hospitals and increase in government support on research activities and other related trends adopted by the market players.
The key trends in the Intrapartum Monitoring Devices Market are increasing incidence of preterm birth, and a surge in technological advancement in Intrapartum Monitoring Devices.
The total market value of the Intrapartum Monitoring Devices Market is $826 million in 2021.
North America is the largest regional market for Intrapartum Monitoring Devices
The forecast period in the report is from 2022 to 2031.
The top companies that hold the market share in the Intrapartum Monitoring Devices Market are Cardinal Health, Inc., Cooper Companies Inc., General Electric Company, Huntleigh Healthcare Limited, Koninklijke Philips N.V., Laborie, MedGyn products, Inc., Mindchild Medical, Inc., Rocket Medical plc, Stalwart Meditech
The market value of the Intrapartum Monitoring Devices Market in 2022 was $882.17 million.
The base year for the report is 2021.
Yes, Intrapartum Monitoring Devices companies are profiled in the report
Loading Table Of Content...



