Mycoplasma Testing Market Research, 2032
The global mycoplasma testing market size was valued at $0.7 billion in 2022 and is projected to reach $2.2 billion by 2032, growing at a CAGR of 11.7% from 2023 to 2032. Mycoplasma testing refers to the detection and identification of mycoplasma bacteria in biological samples, such as cell cultures and clinical samples. Mycoplasma is a group of small bacteria that lack a cell wall and survive in a variety of environments, including laboratory cell cultures. They can contaminate cell cultures, leading to changes in the behavior and characteristics of the cells, which affects the experimental results of research. Therefore, mycoplasma testing is important in cell culture research and the manufacturing of biological products to ensure the purity of the cultures and prevent contamination.

Market Dynamics
Growth & innovations in the diagnostic industry for the manufacturing of mycoplasma testing products owing to the rise in mycoplasma contaminations in the pharmaceutical and biotechnology industry creates an opportunity for the mycoplasma testing market size. The increase in demand for mycoplasma testing products from the biopharmaceutical industry, the rise in research activities in the biotechnology and biopharmaceutical industries are the key factors contributing to the market growth. For instance, the biopharmaceutical industry is expanding rapidly, and the production of biopharmaceuticals requires strict quality control measures, including mycoplasma testing. This has led to an increase in demand for mycoplasma testing products and services.
The growth of the mycoplasma testing market share is expected to be driven by emerging markets, due to the availability of improved healthcare infrastructure, an increase in unmet healthcare needs, and a rise in the prevalence of mycoplasma contamination. Furthermore, regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have issued guidelines and regulations mandating the testing of biologics and vaccines for mycoplasma contamination. This has led to an increased demand for mycoplasma testing in the biopharmaceutical industry.
In addition, technological advancements, such as the development of faster and more accurate mycoplasma testing methods, and instruments are driving the growth of the mycoplasma testing market share. For instance, VenorGeM Advance Kit offered by Minerva Biolabs GmbH is a highly sensitive and specific kit for the detection of mycoplasma contamination in cell cultures and other biological matrices. In addition, Microsart Mycoplasma qPCR Detection Assay Kits offered by Sartorius AG, are rapid and guideline-compliant products for mycoplasma detection. These kits are highly specific, and sensitive, and provide fast results in pharmaceutical quality control processes.
Furthermore, an increase in demand for biologics and biosimilars drives the growth of the market. Mycoplasma contamination can occur during the manufacturing process of biologics and biosimilars, which can lead to compromised product quality, reduced efficacy, and potential patient safety risks. As the demand for biologics and biosimilars continues to increase, the need for effective mycoplasma testing solutions also rises, which, in turn, contributes to market growth. For instance, as of April 2021, the Food and Drug Administration (FDA), has approved 29 biosimilars, and 20 biosimilars have been launched in the U.S. Moreover, an increase in promotional activities by manufacturers and growth in awareness for mycoplasma testing is expected to fuel their adoption in the near future.
On the other hand, the high cost of mycoplasma testing kits and instruments and the scarcity of skilled lab technicians limit the adoption of mycoplasma testing products and negatively affect the market growth. For instance, the price of SouthernBiotech’s Mycoplasma Detection Kit is about $200-250. In addition, the cost of mycoplasma testing for patient specimens such as tissue, blood, and other secretions is around $210. Thus, the high cost of mycoplasma testing products owing to their specialized kits and reagents is anticipated to hamper the growth of the market.
The outbreak of COVID-19 has disrupted workflows in the biotechnology sector around the globe. The global mycoplasma testing market growth experienced a decline in 2020 due to the disruption of supply chains and logistics caused by the pandemic has led to delays in the delivery of mycoplasma testing products and services. This has affected the biopharmaceutical companies to perform mycoplasma testing and has led to delays in the development and production of biologics and biosimilars. On the other hand, the development and production of COVID-19 vaccines and therapeutics require rigorous mycoplasma testing to ensure product safety and efficacy. Therefore, the demand for mycoplasma testing solutions increased during the COVID-19 pandemic.
Global Mycoplasma Testing Market Segmental Overview
The mycoplasma testing market is segmented into product, technology, application, end-user, and region. By product, the market is categorized into instruments, kits & reagents, and services. On the basis of technology, the market is segregated into PCR, ELISA, Microbial Culture Techniques, and Enzymatic Methods. By application, the market is classified into cell line testing, virus testing, end-of-production cell testing, and others. By end user, the market is segmented into academic research institutes, cell banks, contract research organizations, pharmaceutical & biotechnology companies, and others. Region-wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
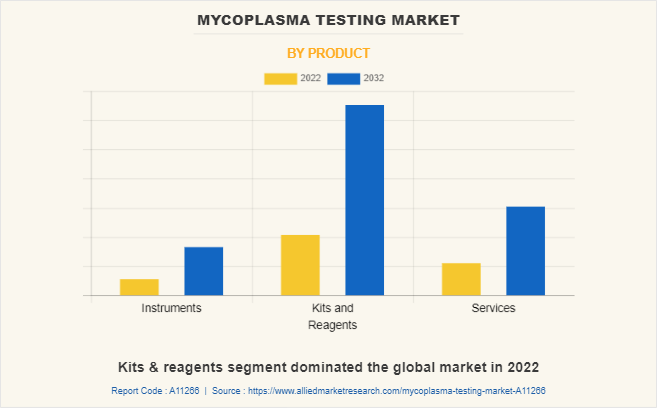
By Product
The mycoplasma testing market is segmented into instruments, kits & reagents, and services. The kits & reagents segment dominated the global market in 2022, and it is the fastest growing segment during the forecast period, owing to the increase in the use of kits and reagents for mycoplasma detection in biotechnology and other research organizations as it is easy to use, economical as compared with other mycoplasma testing instruments.
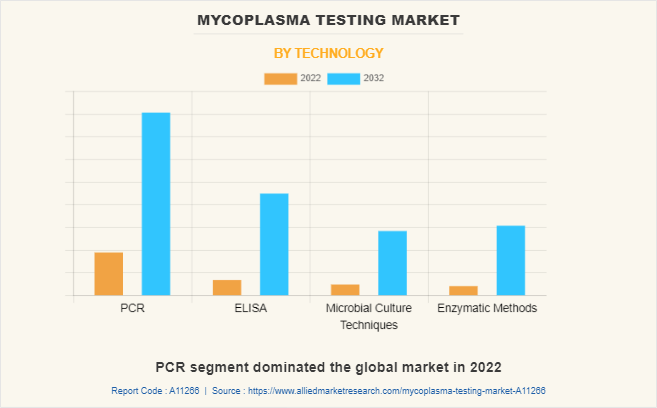
By Technology
The mycoplasma testing market is segregated into PCR, ELISA, microbial culture techniques, and enzymatic methods. The PCR segment dominated the global market in 2022 and is anticipated to continue this trend during the forecast period. This is attributed to the increase in the adoption of PCR-based mycoplasma testing kits as it offers rapid and accurate results. Furthermore, various key players offer advanced PCR-based mycoplasma testing kits such as Bio-Rad Laboratories, Inc. offers Vericheck ddPCR Mycoplasma Detection Kit and Merck KGaA offers advanced Venor GeM Mycoplasma Detection Kit. On the other hand, ELISA is the fastest growing segment during the Mycoplasma Testing Market forecast period as it is highly sensitive, detects various mycoplasma species, and provides quick results.
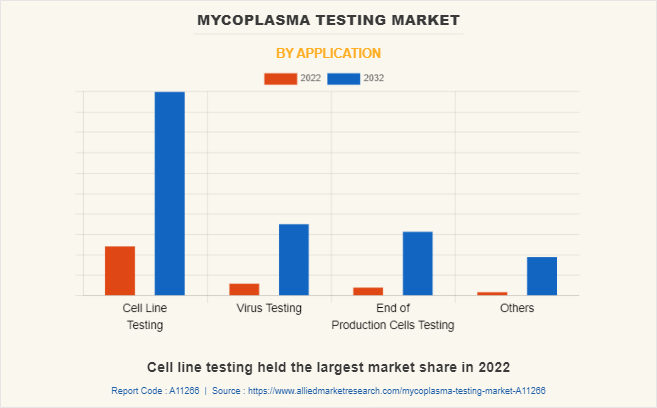
By Application
The mycoplasma testing market is classified into cell line testing, virus testing, end-of-production cell testing, and others. Cell line testing held the largest market share in 2022, and it is the fastest-growing segment during the forecast period, owing to an increase in mycoplasma cell culture contamination and a rise in cell-based research.
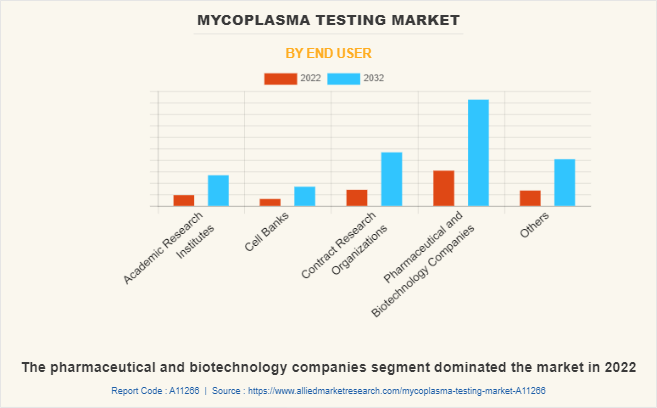
By End User
The mycoplasma testing market is segmented into academic research institutes, cell banks, contract research organizations, pharmaceutical and biotechnology companies, and others. The pharmaceutical and biotechnology companies segment dominated the market in 2022 owing to, a rise in research and development of new biologics for which mycoplasma testing is mandatory and an increase in the adoption of mycoplasma testing products in biotechnology and pharmaceutical industries.
By Region
The mycoplasma testing market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for a major share of the mycoplasma testing market in 2022 and is expected to maintain its dominance during the forecast period. The presence of several major players, such as Thermo Fischer Scientific Inc., BioMérieux, and Eurofins Scientific, and advancement in mycoplasma testing technology of mycoplasma testing in the region drive the growth of the market. In addition, the rise in incidences of mycoplasma contaminations in cell cultures and other biological products has increased the demand for mycoplasma testing products in the region, which propels market growth. In addition, the presence of well-established healthcare infrastructure, high purchasing power, and a rise in the adoption rate of mycoplasma testing products are expected to drive market growth. Furthermore, product launches adopted by the key players in this region boost the growth of the market.
Asia-Pacific is expected to grow at the highest rate during the forecast period. The market growth in this region is attributable to the presence of biopharmaceutical companies in the region as well as growth in the purchasing power of populated countries, such as China and India. Moreover, the rise in demand for biologics and biosimilars and the adoption of high-tech mycoplasma testing products, drive the growth of the market. Furthermore, Asia-Pacific offers profitable opportunities for key players operating in the mycoplasma testing industry, thereby registering the fastest growth rate during the forecast period, owing to the growing infrastructure of industries, rising disposable incomes, as well as the well-established presence of domestic companies in the region. In addition, the rise in contract manufacturing organizations within the region provides a great opportunity for new entrants in this region.
Competition Analysis
Competitive analysis and profiles of the major players in mycoplasma testing, such as ATCC, Bionique Testing Laboratories LLC, Bio-Rad Laboratories Inc., Charles River Laboratories, Eurofins Scientific, F. Hoffmann-La Roche Ltd., InvivoGen, Lonza, Norgen Biotek Corp., and Thermo Fisher Scientific Inc. are provided in this report. There are some important players in the market such as Promocell GmbH, Creative Bioarray, BioMerieux, and others. Major players have adopted product launch and acquisition as key developmental strategies to improve the product portfolio of the mycoplasma testing market.
Recent Product Launches in the Mycoplasma Testing Industry
- BioMérieux, a world leader in the field of in vitro diagnostics launched BIOFIRE MYCOPLASMA, an innovative test for mycoplasma detection in pharmaceutical products used for antibodies, gene therapies, and hormones. BIOFIRE MYCOPLASMA is an advanced, rapid, and easy-to-use molecular biology test. The launch of BIOFIRE MYCOPLASMA will help the company to gain a strong foothold in the mycoplasma testing market.
- Infinity Laboratories, a U.S.-based contract testing laboratory, has expanded its testing capabilities with the launch of mycoplasma testing services. The company is now able to offer both direct culture and PCR-based testing for the detection of mycoplasma contamination in cell culture samples. Infinity Laboratories' mycoplasma testing services are expected to help customers ensure the purity of their cell cultures and comply with regulatory requirements.
Recent Acquisition in the Mycoplasma Testing Market
- Asahi Kasei Medical Co., LTD acquired the Bionique Testing Laboratories LLC a global leader in mycoplasma testing services for the life science and biotherapeutics industry. This acquisition will help the company to expand its biosafety contract testing business.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the mycoplasma testing market analysis from 2022 to 2032 to identify the prevailing mycoplasma testing market opportunity.
- Market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders to make profit-oriented business decisions and strengthen their supplier-buyer network.
- An in-depth analysis of the mycoplasma testing market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global mycoplasma testing market trends, key players, market segments, application areas, and market growth strategies.
Mycoplasma Testing Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 2.2 billion |
| Growth Rate | CAGR of 11.7% |
| Forecast period | 2022 - 2032 |
| Report Pages | 400 |
| By Product |
|
| By Technology |
|
| By Application |
|
| By End User |
|
| By Region |
|
| Key Market Players | InvivoGen, Charles River Laboratories, Thermo Fisher Scientific Inc. , Bionique Testing Laboratories LLC, F. Hoffmann-La Roche Ltd., Bio-Rad Laboratories, Inc. , Norgen Biotek Corp., Eurofins Scientific, Lonza, ATCC |
Analyst Review
Analyst review provides various opinions of the top-level CXOs in the global mycoplasma testing industry. In accordance with several interviews conducted, the mycoplasma testing market is expected to witness healthy growth with a surge in R&D of new biologics and an increase in incidences of mycoplasma cell culture contamination. In addition, the rise in awareness about mycoplasma testing in the biotechnology and biopharmaceutical industries has led to the high adoption of mycoplasma testing products and services, which is expected to significantly boost the growth of the mycoplasma testing market. However, the high cost of mycoplasma testing products and the lack of skilled lab technicians limit the growth of the market. On the other hand, the development of new and advanced technologies for mycoplasma testing is expected to create new opportunities for the mycoplasma testing market. For instance, the development of more accurate and efficient PCR-based assays and automated systems provides rapid and accurate mycoplasma testing results.
The CXOs further added that North America is expected to witness the highest growth, in terms of revenue, owing to an increase in demand for mycoplasma testing services by biopharmaceutical industries, globalization of clinical trials, and launch of new mycoplasma testing service and products are expected to boost the demand for mycoplasma testing in this region. However, Asia-Pacific is expected to register fastest CAGR during the forecast period due to development of healthcare facilities, increase in research activities, and rise in public–private investments in the healthcare sector.
Presence of several major players, and advancements in mycoplasma testing technology of mycoplasma testing are few trends in the Mycoplasma Testing Market report
The cell line testing held the largest market share in 2022
The Mycoplasma Testing Market was valued at $744.80 million in 2022 and is estimated to reach $2,243.10 million by 2032, exhibiting a CAGR of 11.7% from 2023 to 2032.
North America accounted for a major share of the mycoplasma testing market in 2022
Thermo Fisher Scientific Inc., Charles River Laboratories, Lonza, F. Hoffmann-La Roche Ltd., Bio-Rad Laboratories, Inc., and Eurofins Scientific are the top companies in Mycoplasma Testing Market report
Yes, the competitive landscape is included in the Mycoplasma Testing Market report
One of the major restraints in Mycoplasma Testing Market report is the high cost of testing
There are 10 companies profiled in the Mycoplasma Testing Market report
Loading Table Of Content...
Loading Research Methodology...


