Neuroblastoma Drugs Market Statistics, 2032
The global neuroblastoma drugs market size was valued at $0.7 billion in 2022, and is projected to reach $1.3 billion by 2032, growing at a CAGR of 6.2% from 2023 to 2032. The growth of the neuroblastoma drugs is driven by a rise in R&D activities and rise in incidences of neuroblastoma. For instance, according to the American Society of Clinical Oncology (ASCO), neuroblastoma accounted for 6% of all childhood cancers in the U.S. and about 90% of neuroblastoma is found in children younger than 5. Thus, rise in cases of neuroblastoma led to an increase in demand for neuroblastoma drugs.
Key Takeaways
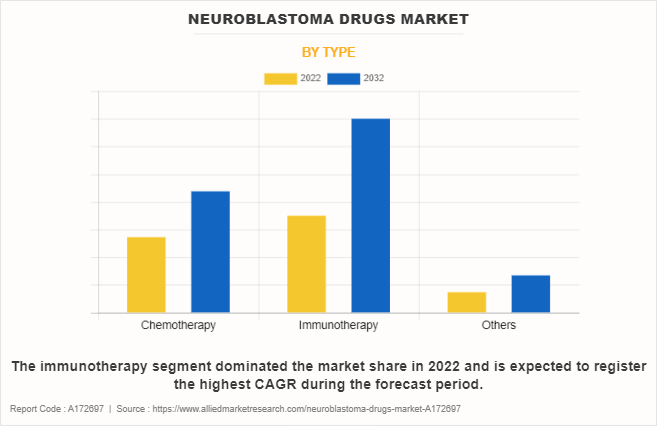
- By type, the immunotherapy segment dominated the global market in 2022.
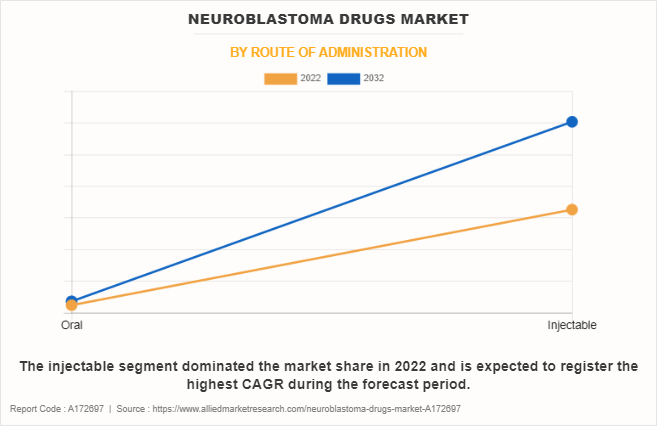
- By route of administration, the injectable segment dominated the global market in 2022.
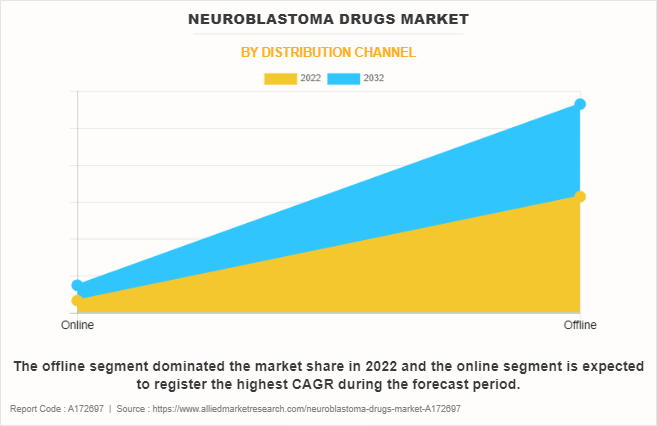
- By distribution channel, the offline segment dominated the market in terms of revenue in 2022.
- By region, North America dominated the market in terms of revenue in 2022. However, Asia-Pacific is anticipated to grow at the highest CAGR during the forecast period.
Neuroblastoma is a type of cancer that commonly occurs in young children, typically under the age of five. It originates in nerve tissue, specifically in the adrenal glands, which are located on top of the kidneys. It arises from immature nerve cells, called neuroblasts, and it is known for its variability in terms of both presentation and prognosis. Symptoms of neuroblastoma can vary widely and may include a lump or swelling in the abdomen, changes in bowel habits, bone pain, fatigue, and, in some cases, skin changes.
Market Dynamics
The increase in incidence of neuroblastoma serves as a primary driver propelling the neuroblastoma drugs market growth. Over recent years, there has been a noticeable rise in the diagnosis of neuroblastoma, particularly among pediatric populations. This upward trend in incidence has heightened the demand for effective and advanced therapeutic interventions. As more cases are identified through improved diagnostic capabilities and heightened awareness, pharmaceutical companies and researchers are prompted to intensify their efforts in developing innovative neuroblastoma drugs, thereby supporting the market growth.
In addition, rise in R&D activities is driving the growth of the neuroblastoma drugs industry, leading to the discovery and development of innovative therapies. The continuous progress in understanding the intricate molecular and genetic mechanisms underlying neuroblastoma has led to the identification of novel therapeutic targets and the development of more effective treatment strategies. For instance, in March 2023, Aptorum Group, a clinical-stage biopharmaceutical company, announced the completion of an end of phase 1 (EOP1) meeting with the U.S. Food and Drug Administration. SACT-1 is a repurposed small molecule drug formulated in oral suspension targeting neuroblastoma in pediatric patients and has also received orphan drug designation previously from the U.S. FDA.
Furthermore, government initiatives and funding play a crucial role in driving the growth of the neuroblastoma drugs market. Recognizing the urgency and severity of pediatric cancers, including neuroblastoma, governments around the world have increasingly allocated substantial resources to support research, development, and accessibility of innovative treatments. These initiatives create a favorable environment for pharmaceutical companies, research institutions, and healthcare organizations to collaborate and advance neuroblastoma drug development, which provides neuroblastoma drugs market opportunity.
In contrast, adverse side effects associated with neuroblastoma drugs pose a significant restraint on market growth. The use of therapies such as chemotherapy, a conventional approach in neuroblastoma treatment, often leads to notable side effects that can impact patients' quality of life. Pediatric patients may experience long-term consequences, including developmental delays and compromised organ function. The prospect of adverse reactions creates reluctance among both healthcare providers and patients, influencing treatment decisions and compliance, which may negatively impact the neuroblastoma drugs market growth.
During a recession, the market may experience a multifaceted impact. Economic downturns lead to budget constraints in healthcare systems, potentially reducing funding for R&D activities of new drugs. Patients and healthcare providers may face financial challenges, impacting their ability to afford or prescribe expensive neuroblastoma drugs. Despite these challenges, the market continues to experience moderate revenue growth owing to urgency and critical nature of pediatric oncology is projected to drive increased attention and investment in R&D. Thus, the neuroblastoma drugs market is moderately impacted by recession.
Segments Overview
The neuroblastoma drugs market analysis is segmented into type, route of administration, distribution channel, and region. By type, the market is categorized into chemotherapy, immunotherapy, and others. By route of administration, the market is classified into oral and injectable. By distribution channel, the market is classified into offline and online. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
By Type
The immunotherapy segment dominated the global neuroblastoma drugs market share and also is expected to register the highest CAGR during the neuroblastoma drugs market forecast period, owing to increasing understanding and research advancements, immunotherapy demonstrates potential for more effective and durable responses. In addition, ongoing clinical trials and collaborative efforts amplify the momentum of immunotherapy, positioning it as a transformative approach in the evolving landscape of neuroblastoma treatment, thereby driving the segment growth.
By Route of Administration
The injectable segment dominated the global neuroblastoma drugs market share in 2022 and is expected to register the highest CAGR during the forecast period, owing to its efficacy, convenience, and the ability to deliver precise doses of neuroblastoma drugs. In addition, injectable formulations offer a reliable and direct administration route, ensuring rapid absorption and bioavailability, particularly crucial in pediatric oncology, further supporting the segment growth.
By Distribution Channel
The offline segment held the largest neuroblastoma drugs market size in 2022, owing to established trust and accessibility offered by healthcare facilities, ensuring widespread availability of neuroblastoma drugs. However, the online segment is expected to register the highest CAGR during the forecast period, owing to rise in trend of online healthcare, enabling patients to access information, consult with healthcare professionals remotely, and order medications conveniently.
By Region
Neuroblastoma drugs industry is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for a major share of the neuroblastoma drugs market in 2022 and is expected to maintain its dominance during the forecast period.
The presence of several major players, such as Pfizer, Inc., AstraZeneca Plc, Bristol-and Myers Squibb Company in the region drive the growth of the market. In addition, high healthcare expenditure, government initiatives and policies in supporting pediatric oncology research and the development of innovative treatment options for neuroblastoma, are driving the demand for neuroblastoma drugs.
Asia-Pacific offers profitable opportunities for key players operating in the neuroblastoma drugs market, thereby registering the fastest growth rate during the forecast period, owing to rise in the incidence of neuroblastoma among pediatric populations, necessitating increased focus on effective treatment options. In addition, the growth in awareness of neuroblastoma and increase in healthcare expenditure are creating a demand for advanced and targeted drug therapies.
Competition Analysis
Competitive analysis and profiles of the major players in the neuroblastoma drugs market, such as Recordati Group, AstraZeneca plc, Bristol-Myers Squibb Company, Cellectar Biosciences, Inc., Eli Lilly and Company, MacroGenics Inc, Pfizer, Inc., Teva Pharmaceutical Industries Ltd., United Therapeutics Corporation, and Y-mabs Therapeutics, Inc., are provided in this report. Major players have adopted product approval, partnership, agreement, and acquisition as key developmental strategies to improve the product portfolio of the neuroblastoma drugs market.
Recent Developments in the Neuroblastoma Drugs Market
- In May 2023, Y-mAbs Therapeutics, Inc., a commercial-stage biopharmaceutical company announced that the Brazilian Health Regulatory Agencynhas granted marketing authorization for DANYELZA (naxitamab-gqgk) 40mg/10mL injection. DANYELZA will be marketed in Brazil by Y-mAbs partner Adium Pharma S.A.
- In December 2022, Y-mAbs Therapeutics, Inc. announced that the National Medical Products Administration in China has granted DANYELZA (naxitamab-gqgk) 40mg/10ml conditional approval. DANYELZA will be marketed in China by Y-mAbs™ partner SciClone Pharmaceuticals (Holdings) Limited.
- In August 2022, Y-mAbs Therapeutics, Inc., a commercial-stage biopharmaceutical company and Takeda Israel, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, announced that the Israeli Ministry of Health has approved DANYELZA in Israel for the treatment, in combination with granulocyte-macrophage colony-stimulating factor, of pediatric patients 1 year of age and older and adult patients with relapsed or refractory high-risk neuroblastoma.
- In August 2021, BeiGene, Ltd. and EUSA Pharma (UK), Ltd a part of Recordati announced that the China National Medical Products Administration (NMPA) has granted QARZIBA (dinutuximab beta) conditional approval for the treatment of high-risk neuroblastoma in patients aged 12 months and above who have previously received induction chemotherapy and achieved at least a partial response, followed by myeloablative therapy and stem cell transplantation.
- In August 2021, United Therapeutics Corporation announced it is joining forces with former NFL player Devon Still and his daughter Leah, a survivor of high-risk neuroblastoma, to launch the educational initiative "Braving NeuroBLASToma" for educating about the rare cancer affecting immature nerve cells called neuroblasts.
- In March 2023, Recordati completed the acquisition of EUSA Pharma (UK), a global specialty pharmaceutical company focused on rare and niche oncology diseases.
- In February 2023, Y-mAbs Therapeutics, Inc. a commercial-stage biopharmaceutical company announced that the European Medicines Agency has agreed to its proposed pediatric investigation plan for naxitamab. The decision follows a positive opinion from Pediatric Committee. Naxitamab is being developed by Y-mAbs for the treatment of patients with relapsed/refractory high-risk neuroblastoma, which is the indication targeted by the PIP, as well as osteosarcoma.
- In February 2022, Y-mAbs Therapeutics, Inc., a commercial-stage biopharmaceutical company announced that it has entered into a distribution agreement with WEP Clinical Ltd. in connection with an early access program for DANYELZA (naxitamab-gqgk) 40mg/10mL Injection in Europe.
- In May 2021, Y-mAbs Therapeutics, Inc., a commercial-stage biopharmaceutical company announced that it has entered into an exclusive distribution agreement with Adium Pharma S.A. to be the exclusive distributor in Latin America of the Company antibodies, DANYELZA (naxitamab-gqgk) for the treatment of patients with relapsed/refractory high-risk neuroblastoma.
- In January 2020, EUSA Pharma and BeiGene, Ltd. announced that they have entered into an exclusive development and commercialization agreement for the orphan biologic products SYLVANT (siltuximab) and QARZIBA (dinutuximab beta) in Greater China.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the market from 2022 to 2032 to identify the prevailing neuroblastoma drugs market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the neuroblastoma drugs market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global neuroblastoma drugs market trends, key players, market segments, application areas, and market growth strategies.
Neuroblastoma Drugs Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 1.3 billion |
| Growth Rate | CAGR of 6.2% |
| Forecast period | 2022 - 2032 |
| Report Pages | 256 |
| By Type |
|
| By Route of Administration |
|
| By Distribution Channel |
|
| By Region |
|
| Key Market Players | Bristol-Myers Squibb Company, Pfizer Inc., United Therapeutics Corporation, Y-mabs Therapeutics, Inc., AstraZeneca plc, Eli Lilly and Company, Recordati Group, Teva Pharmaceutical Industries Ltd., MacroGenics Inc., Cellectar Biosciences, Inc. |
Analyst Review
This section provides various opinions of global neuroblastoma drugs market. The global neuroblastoma drugs market is expected to exhibit high growth potential attributable to surge in cases of neuroblastoma, rise in investments for R&D activities, upsurge in demand for sophisticated healthcare facilities and rise in healthcare expenditure drive the market growth. However, side effects of drugs and high cost of cancer treatment in some regions limit the growth of the neuroblastoma drugs market.?
In addition, key players have adopted various strategies to strengthen their foothold in the competitive market. In addition, rise in incidences of neuroblastoma and increased awareness of neuroblastoma among healthcare professionals, patients, and the general public contribute to early diagnosis and treatment which further propels the market growth.
Furthermore, North America dominated the market share, in terms of revenue, owing to surge in cases of cancer, easy availability of drugs, high healthcare expenditure, and strong presence of key players in this region. However, Asia-Pacific is anticipated to witness notable growth owing to initiatives by the government & non-governmental organizations (NGOs) to promote awareness regarding neuroblastoma and increase in public–private investments in the healthcare sector.
The total market value of neuroblastoma drugs market is $0.7 billion in 2022.
The market value of neuroblastoma drugs market in 2032 is $1.3 billion.
The forecast period for neuroblastoma drugs market is 2023 to 2032.
The base year is 2022 in neuroblastoma drugs market.
United Therapeutics Corporation, Pfizer, Inc., Recordati Group, Y-mAbs Therapeutics, Inc, and Bristol-Myers Squibb Company held a high market position in 2022.
Immunotherapy segment dominated the market share in 2022, owing to growth in awareness of the potential benefits of immunotherapy, both among healthcare professionals and the public coupled with ongoing R&D activities in this field drives the demand for immunotherapy.
Neuroblastoma is a type of cancer that develops from immature nerve cells, or neuroblasts. It typically occurs in the adrenal glands, which are located on top of the kidneys, but it can also originate in nerve tissue along the spine, chest, abdomen, or pelvis.
Loading Table Of Content...
Loading Research Methodology...


