Nitinol-Based Medical Device Market Research, 2035
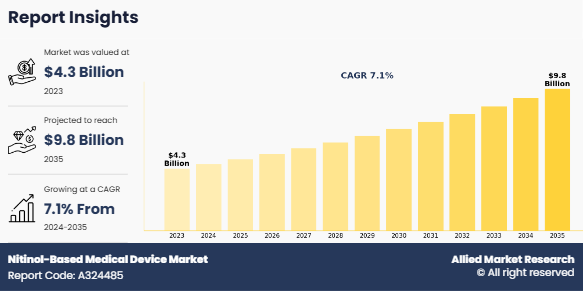
The global nitinol-based medical device market size was valued at $4.3 billion in 2023, and is projected to reach $9.8 billion by 2035, growing at a CAGR of 7.1% from 2024 to 2035. The nitinol-based medical devices market is expanding due to the rising prevalence of chronic diseases. According to the Centers for Disease Control and Prevention (CDC), in 2023, six in ten adults in the U.S. were affected by chronic diseases. This increasing burden drives the demand for advanced medical devices, particularly those leveraging nitinol's unique properties, such as shape memory and flexibility. These devices are widely adopted in cardiovascular, orthopedic, and dental applications, addressing the growing need for effective and minimally invasive treatments.
Nitinol, a nickel-titanium alloy, is known for its unique properties of shape memory and superelasticity, making it an ideal material for medical applications. Shape memory enables nitinol to return to its original shape after deformation, while superelasticity allows it to withstand significant stress without permanent deformation. These characteristics, along with biocompatibility and corrosion resistance, make nitinol suitable for creating advanced medical devices. Nitinol-based medical devices are extensively used in various fields, including cardiovascular, orthopedic, urology, and dentistry. Examples include stents, guidewires, catheters, retrieval baskets, orthodontic archwires, and orthopedic staples. These devices enhance the precision and success rates of minimally invasive procedures, offering improved patient outcomes. The growing adoption of nitinol in medical technology reflects its importance in addressing complex healthcare challenges.
Key Takeaways
- On the basis of product type, the stents segment dominated the market share in 2023. However, the others segment is anticipated to grow at the highest CAGR during the nitinol-based medical device market forecast period.
- On the basis of application, the cardiovascular segment dominated the market share in 2023. However, the others segment is anticipated to grow at the highest CAGR during the forecast period.
- On the basis of end user, the Hospitals segment dominated the market share in 2023. However, the ambulatory surgical centers segment is anticipated to grow at the highest CAGR during the forecast period.
- Region wise, North America generated the largest revenue in 2023. However, Asia-Pacific is anticipated to grow at the highest CAGR during the forecast period.
Market Dynamics
The nitinol-based medical device market growth is significantly shaped by drivers such as the rising prevalence of chronic diseases, which include cardiovascular disorders, urological conditions, and orthopedic ailments. Chronic diseases, particularly cardiovascular conditions, remain a leading cause of mortality and morbidity worldwide. For instance, coronary artery disease and peripheral arterial disease affect millions globally, leading to a higher demand for advanced medical interventions. Nitinol-based devices, such as stents, guidewires, and retrieval baskets, are preferred in these treatments due to their unique properties, including flexibility, biocompatibility, and durability. These devices are indispensable in enabling precise and efficient minimally invasive procedures, making them a cornerstone in addressing the growing burden of chronic diseases.
Advancements in medical technology further act as a major growth driver for this market. Continuous innovation in nitinol-based devices, such as self-expanding stents, drug-eluting devices, and shape-memory retrieval baskets, demonstrates their versatility and adaptability in various medical applications. These cutting-edge solutions have revolutionized minimally invasive surgeries by offering improved outcomes, shorter recovery times, and reduced risks of complications. For example, the integration of hydrophilic coatings enhances device maneuverability, while MRI-compatible designs ensure broader applicability in diagnostics and treatment. Enhanced fatigue resistance and superelasticity further ensure device reliability and longevity. Collectively, these advancements bolster the adoption of nitinol-based devices across diverse medical fields, driving sustainednitinol-based medical device market growth.
On the other hand, the high cost of nitinol-based devices remains a significant restraint. The complex manufacturing processes, including precision alloying and shape-memory programming, drive up production costs, making these devices less accessible in cost-sensitive regions. For instance, while nitinol devices offer long-term benefits, their upfront cost can deter adoption, particularly in developing countries where healthcare budgets are constrained.
Stringent regulatory requirements also hinder market growth. Medical devices must meet rigorous standards for safety, efficacy, and quality, leading to prolonged approval timelines. These regulatory challenges increase development costs for manufacturers and delay the availability of new products in the market. For example, nitinol's unique properties, such as its shape memory and superelasticity, require extensive testing to ensure reliability, further complicating the approval process.
Despite these challenges, the growing preference for minimally invasive procedures acts as a substantial driver for the market. Patients and healthcare providers increasingly favor minimally invasive techniques due to their reduced recovery times, lower complication rates, and improved patient comfort. Nitinol-based devices play a pivotal role in enabling these procedures, particularly in cardiovascular interventions, urological surgeries, and neurological treatments. For instance, nitinol stents and guidewires are crucial in delicate surgeries, allowing precise navigation through intricate anatomical structures.
The increased adoption of outpatient settings, such as ambulatory surgical centers (ASCs), further supports market expansion. Outpatient facilities offer cost-effective solutions for minimally invasive procedures, reducing hospital stays and overall healthcare costs. With advancements in nitinol-based devices enabling safer and quicker procedures, ASCs have emerged as a preferred choice for patients and insurers alike. This shift has amplified demand for nitinol products, particularly for treatments in urology, orthopedics, and interventional radiology.
Segmental Overview
The global nitinol-based medical device industry is segmented into product type, application, end user, and region. On the basis of product type, it is segmented into stents, guidewires, retrivers device, catheters, and others. On the basis of application, the market is classified into cardiovascular, dentistry, urology, and other. On the basis of end user, it is classified into hospitals, ambulatory surgical centers, and others. On the basis of region, the market is segmented into North America, Europe, Asia-Pacific, and LAMEA.
By Product Type
By product type, the retrievers device segment dominated the nitinol-based medical device market share in 2023. This is attributed to their superior flexibility, biocompatibility, and shape memory properties. These features enhance precision in minimally invasive procedures, improving patient outcomes. Increasing demand for advanced retrieval solutions in urology, gastroenterology, and vascular applications further accelerates market expansion.
However, the catheters segment is expected to register the highest CAGR during the forecast period owing to its superior flexibility, kink resistance, and shape memory properties. These features enhance navigation through complex anatomies, improving procedural efficiency and patient outcomes. Additionally, rising demand for minimally invasive procedures and increasing cardiovascular and urological interventions further drive growth.
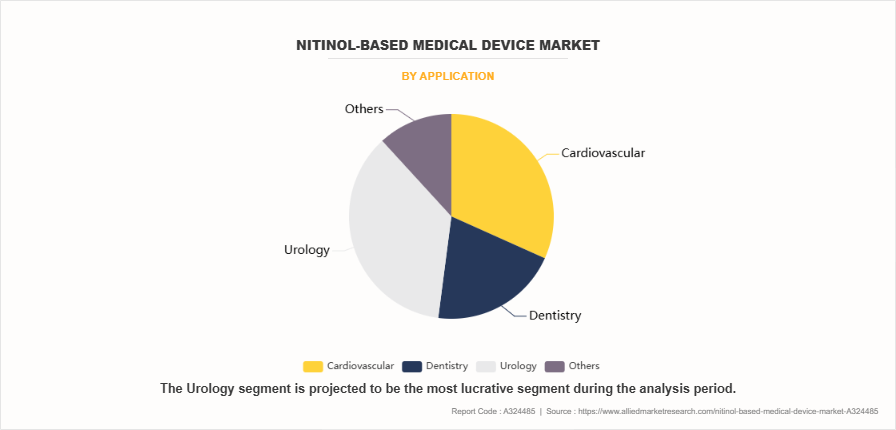
By Application
By application, the cardiovascular segment dominated the nitinol-based medical device market share in 2023. This was attributed to the widespread prevalence of heart-related conditions, boosting the demand for nitinol-based stents, guidewires, and catheters. These devices facilitate minimally invasive procedures, offering enhanced outcomes. Innovations such as drug-eluting stents and self-expanding devices, along with the growing adoption of interventional cardiology techniques, played a pivotal role in establishing this segment's dominance.
However, the others segment is expected to register the highest CAGR during the forecast period owing to the increasing use of nitinol-based devices across various fields. Rising demand for minimally invasive solutions in neurosurgery and interventional radiology, advancements in orthopedic implants, and the growing application of flexible, biocompatible devices in complex surgical procedures contribute to this growth. Additionally, improvements in healthcare infrastructure are further propelling the adoption of these advanced devices across diverse applications.

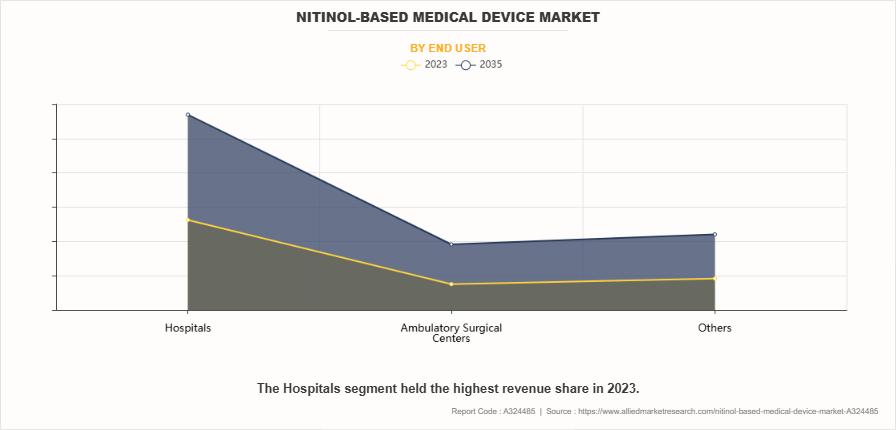
By End User
By end user, the hospitals segment dominated the nitinol-based medical device market size in 2023, owing to the high volume of surgical and interventional procedures performed in these facilities. Hospitals are the primary users of nitinol-based devices, such as stents, guidewires, and catheters, for managing cardiovascular, urological, and orthopedic conditions. Their advanced infrastructure, skilled medical professionals, and capacity to handle complex cases solidify their market dominance. Additionally, the rise in patient admissions for minimally invasive procedures has significantly contributed to this segment's growth.
However, the ambulatory surgical centers segment is expected to register the highest CAGR during the forecast period owing to the increasing preference for cost-effective, minimally invasive procedures performed in outpatient settings. ASCs offer advantages like shorter recovery times, reduced hospital stays, and lower overall costs, making them a preferred choice for patients and insurers. The growing adoption of advanced nitinol-based devices for urology, cardiovascular, and orthopedic treatments further fuels this segment's expansion.
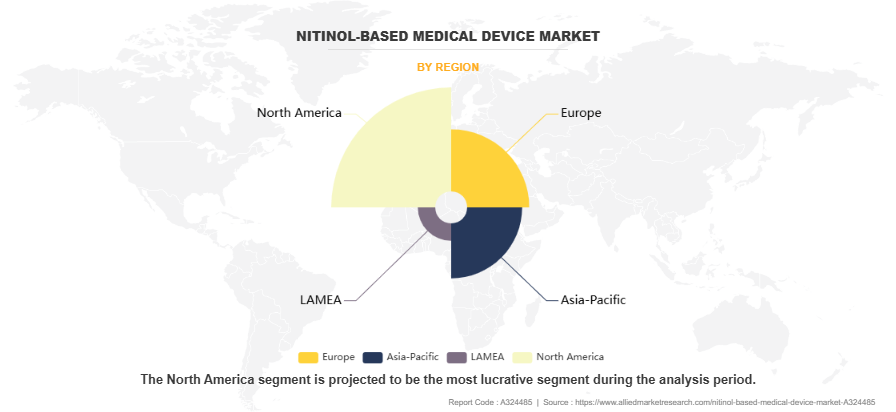
By Region
Region wise, North America was the largest shareholder in the nitinol-based medical device market in 2023, owing to the high prevalence of chronic diseases such as cardiovascular and urological disorders. The region benefits from advanced healthcare infrastructure, extensive adoption of minimally invasive procedures, and a strong presence of key market players. Additionally, continuous technological advancements and rising patient awareness further fueled the demand for nitinol-based medical devices in this region.
However, Asia-Pacific is anticipated to register the highest CAGR during the forecast period owing to rapid improvements in healthcare infrastructure, rising healthcare expenditures, and increasing awareness of advanced medical technologies. Growing populations, along with rising incidences of chronic diseases, particularly cardiovascular and urological conditions, drive demand for nitinol-based devices. Additionally, expanding medical device manufacturing capabilities and increasing healthcare accessibility in emerging economies contribute to this segment’s robust growth.
Competition Analysis
Competitive analysis and profiles of the major players in the Arthrex Inc, Boston Scientific Corporation, Zimmer Biomet, Becton, Dickinson & Company, Terumo Corporation, W.L. Gore & Associate Inc., Medtronic Plc, Cardinal Health (Cordis), 3M company, and Cook Medical. The key players have adopted product launch as the key strategies for expansion of their product portfolio.
Recent Developments in Nitinol-Based Medical Device Industry
- In September 2020, Veryan Medical launched the BioMimics 3D Vascular Stent System in the U.S. This self-expanding, nitinol-based stent features a helical center-line design, offering improved clinical outcomes, as demonstrated by a pivotal study with a three-year follow-up.
- In March 2024, BD (Becton, Dickinson and Company), a global leader in medical technology, initiated the AGILITY investigational device exemption (IDE) study to evaluate the safety and effectiveness of its BD Vascular Covered Stent for treating Peripheral Arterial Disease (PAD). This advanced device is a self-expanding, low-profile nitinol implant encapsulated with polytetrafluoroethylene, designed for precise deployment through a controlled delivery system.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the nitinol-based medical device market analysis from 2023 to 2035 to identify the prevailing nitinol-based medical device market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the nitinol-based medical device market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global nitinol-based medical device market trends, key players, market segments, application areas, and market growth strategies.
Nitinol-Based Medical Device Market Report Highlights
| Aspects | Details |
| Market Size By 2035 | USD 9.8 billion |
| Growth Rate | CAGR of 7.1% |
| Forecast period | 2023 - 2035 |
| Report Pages | 285 |
| By Product Type |
|
| By Application |
|
| By End User |
|
| By Region |
|
| Key Market Players | Becton, Dickinson, and Company, Arthrex Inc, Zimmer Biomet Holding, Inc., Boston Scientific Corporation, W.L. Gore & Associate Inc, Solventum, Terumo Corporation, Cordis, Cook Group, Medtronic plc |
Analyst Review
This section presents insights from top-level CXOs in the nitinol-based medical devices market. According to CXOs, the market is primarily driven by the increasing prevalence of chronic diseases, advancements in minimally invasive surgeries, and the superior properties of nitinol, such as shape memory and superelasticity. They emphasized that innovations, including MRI-compatible devices and expanding applications in cardiovascular, orthopedic, and urological treatments, are significant growth factors.
It also highlighted the role of proven clinical outcomes and the adoption of nitinol devices in various surgeries as key contributors to the market's success. However, they noted that high production costs and the technical complexity of manufacturing nitinol devices could pose challenges to market growth. Regulatory compliance and quality assurance were also flagged as potential hurdles.
North America dominates the market, attributed to its advanced healthcare infrastructure, robust R&D investments, and high adoption of cutting-edge medical technologies. CXOs foresee the Asia-Pacific region experiencing the highest CAGR during the forecast period, driven by increasing healthcare demand, a rising focus on minimally invasive procedures, and supportive government initiatives aimed at modernizing healthcare systems and boosting medical device manufacturing capabilities.
The total market value of Nitinol-based medical device Market was $ 4.3 billion in 2023.
The market value of Nitinol-based medical device Market is projected to reach $ 9.8 billion by 2035.
The forecast period for Nitinol-based medical device Market is 2024 to 2035
The base year is 2023 in Nitinol-based medical device Market.
Nitinol, a unique alloy primarily composed of nickel and titanium, is renowned for its exceptional properties, including shape memory and superelasticity. These characteristics allow nitinol to "remember" its original shape when exposed to specific temperatures, making it an ideal material for medical applications. The manufacturing process of nitinol-based medical devices involves melting and alloying nickel and titanium in precise proportions, followed by heat treatment to induce the desired shape memory and superelastic characteristics.
Loading Table Of Content...
Loading Research Methodology...



