North America Prosthetic Heart Valve Market Research, 2033
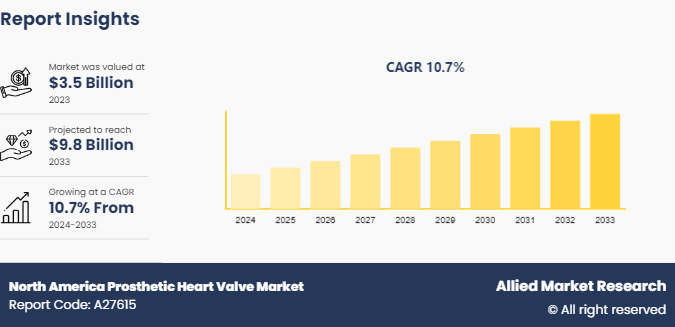
The North America prosthetic heart valve market size was valued at $3.5 billion in 2023, and is projected to reach $9.8 billion by 2033, growing at a CAGR of 10.7% from 2024 to 2033. A prosthetic heart valve is an artificial device implanted in the heart to replace a damaged or diseased natural valve, ensuring proper blood flow through the heart and to the rest of the body. The market focuses on the development, manufacturing, and sales of artificial heart valves used to replace damaged or diseased valves in patients with cardiovascular conditions. North America prosthetic heart valve market trends include addressing the growing prevalence of heart valve diseases and rise in advancements in technology.
Key Market Dynamics
The North America prosthetic heart valve market growth driven by the increasing prevalence of cardiovascular diseases, advancements in minimally invasive technologies such as TAVR, heightened awareness and early diagnosis of valvular heart diseases, and supportive reimbursement and regulatory environments. In addition, the increase in the demand for prosthetic heart valves, rise in geriatric population, which is more susceptible to heart conditions, further drives the market growth during North America prosthetic heart valve market forecast period. Furthermore, advancements in medical technologies, such as the development of minimally invasive surgical procedures such as transcatheter aortic valve replacement (TAVR) , have made heart valve surgeries safer and more accessible. These technological innovations, coupled with improved patient outcomes, significantly boost the North America prosthetic heart valve market share.
However, stringent regulatory approvals and the lengthy process of clinical trials can also delay the introduction of new products, thereby limiting the North America prosthetic heart valve market growth. On the contrary, growing adoption of transcatheter aortic valve replacement (TAVR) procedures, which provide a minimally invasive alternative to open-heart surgery thereby provides market opportunity. The development of next-generation valves with enhanced durability and reduced complications presents significant market potential. Additionally, increased investment in R&D for innovative valve technologies, coupled with favorable regulatory support and reimbursement policies, can also drive North America prosthetic heart valve market size.
Market Segmentation
The North America prosthetic heart valve industry is segmented on the basis of product and country. On the basis of the product, the market is categorized into mechanical heart valve, tissue heart valve, and transcatheter heart valve. The tissue heart valve segment is further classified into stented tissue heart valve and stent less tissue heart valve. The transcatheter heart valve segment dominated the North America prosthetic heart valve market share in 2023, owing to ongoing advancements in transcatheter valve technologies, which enhance safety and effectiveness. In addition, increased adoption in outpatient and ambulatory settings further drives its dominance. On the basis of country the market is classified into U.S., Canada, and Mexico.
Industry Trends
- The American Heart Association advocates for robust funding for the Center for Disease Control and Prevention (CDC's) Heart Disease and Stroke Prevention Programs, including heart disease and stroke prevention, Million Hearts 2022, and WISEWOMAN. These programs work to prevent, manage and reduce heart disease and stroke across all 50 states and District of Columbia. These programs increase awareness and early detection of cardiovascular conditions, leading to earlier diagnosis and intervention, which often involves prosthetic valves. Enhanced funding supports research and public health efforts, potentially leading to the development of improved valve technologies and treatment protocols thereby supporting the market growth.
Competitive Landscape
The key players operating in the North America prosthetic heart valve industry are Abbott Laboratories, Artivion, Inc, Anteris Technologies Ltd, Boston Scientific Corporation, Edward Lifesciences Corporation, JenaValve Technology, Inc., Colibri Heart Valve, Medtronic plc, Meril Life Sciences Pvt. Ltd., and ShockWave Medical, Inc. These players have adopted various developmental strategies to stay competitive in the market. For instance, in March 2023, Abbott announced that the U.S. Food and Drug Administration (FDA) has approved the company's Epic Max stented tissue valve to treat people with aortic regurgitation or stenosis. This device is the latest addition to Abbott's Epic surgical valve platform which has a decades-long history of safety and strong clinical outcomes, with an optimized design to further improve valve blood flow.
Key Benefits for Stakeholders
- The report provides a comprehensive analysis of the current market estimations through 2024-2033, which would enable the stakeholders to capitalize on prevailing market opportunities.
- Major countries are mapped according to their revenue contribution to the North America prosthetic heart valve market.
- In-depth analysis of the North America prosthetic heart valve market segmentation assists to determine the prevailing market opportunities.
- Identify key players and their strategic moves in North America prosthetic heart valve market.
- Assess and rank the top factors that are expected to affect the growth of North America prosthetic heart valve market.
- Analyze the market factors in various countries and understand business opportunities.
- Player positioning provides a clear understanding of the present position of key market players.
North America Prosthetic Heart Valve Market Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 9.8 Billion |
| Growth Rate | CAGR of 10.7% |
| Forecast period | 2024 - 2033 |
| Report Pages | 110 |
| By Product |
|
| By Country |
|
| Key Market Players | JenaValve Technology, Inc., Colibri Heart Valve, Meril Life Sciences Pvt. Ltd., Abbott Laboratories, Edward Lifesciences Corporation, Anteris Technologies Ltd, Medtronic plc, ShockWave Medical, Inc., Boston Scientific Corporation, Artivion, Inc. |
The North America prosthetic heart valve market is projected to grow at a CAGR of 10.7 % from 2024 to 2033
Abbott Laboratories, Artivion, Inc., Anteris Technologies Ltd, Boston Scientific Corporation, Edward Lifesciences Corporation, JenaValve Technology, Inc., Colibri Heart Valve, Medtronic plc, Meril Life Sciences Pvt. Ltd., ShockWave Medical, Inc. are the leading players in North America Prosthetic Heart Valve Market
1. The report provides a comprehensive analysis of the current market estimations through 2024-2033, which would enable the stakeholders to capitalize on prevailing market opportunities. 2. Major countries are mapped according to their revenue contribution to the North America prosthetic heart valve market. 3. In-depth analysis of the North America prosthetic heart valve market segmentation assists to determine the prevailing market opportunities. 4. Identify key players and their strategic move
For analysis, the North America prosthetic heart valve market is segmented into on the basis of product and country.
Loading Table Of Content...



