Skeletal Dysplasia Market Research, 2031
The global skeletal dysplasia market was valued at $1,638.1 million in 2021, and is projected to reach $2,360.51 million by 2031, growing at a CAGR of 3.7% from 2022 to 2031. Skeletal dysplasia is a rare disease that manifests itself in over 450 different forms. It is often regarded as Osteochondrodysplasias. It affects the growth and development of bone and cartilage. This disorder can be severe enough to cause newborn death or abnormal adult development. Skeletal dysplasia is a rare disorder that is difficult to identify. Some of the symptoms include slow growth, abnormalities including a large head, short upper arms or legs, twisted bones, arthritis, or joint pain. Infants are extremely affected by the disease, and early identification helps to increase awareness. This condition is brought on by defective genes, which can either be inherited from ancestors or changed while a fetus is developing. It is also quite difficult to locate the mutant gene. Therefore, multinational corporations are actively investing in the research and development of therapies for this inherited disorder.
Market Dynamics
Factors that drive the growth of the skeletal dysplasia market include increase in pediatric population, increase in prevalence of skeletal dysplasia and increase in adoption of skeletal dysplasia treatments. For instance, according to a journal published on UpToDate, prevalence of skeletal dysplasia is estimated to be 2.4 per 100,000 births globally every day. Prevalence of skeletal dysplasia during pregnancy is estimated to be 7.5 per 100,000 women screened through ultrasound. Patients with this condition undergo extensive medical and surgical treatments throughout childhood and adulthood. Moreover, commercialization of novel drugs and presence of pipeline products further propel market growth. For instance, Amgen Inc. is developing Denosumab, a phase III clinical trial product for the treatment of Osteogenesis Imperfecta (OI). Also known as brittle bone disease, OI is a type of skeletal dysplasia causing extremely short limbs.
Furthermore, the presence of a plethora of products in the pipeline and high market potential in untapped emerging economies are anticipated to serve as attractive skeletal dysplasia market opportunity during the forecast period. Moreover, growth in medical tourism in developing economies and rapid implementation of technology in healthcare industry significantly propels the growth of the market. Furthermore, rising awareness of skeletal dysplasia is also anticipated to propel market growth. For instance, The Fetal Medicine Foundation and SKELDYS.ORG are some of the online portals providing information on the diagnosis and available treatment options for various types of skeletal dysplasia. Additionally, emerging drug therapies for treating the disorder are witnessed to contribute to the skeletal dysplasia market growth over the forecast period.
The COVID-19 pandemic has negative impact on the skeletal dysplasia market owing to investments in research and development of novel growth hormone products that target skeletal dysplasia as well as funding by various companies engaged in the research and development sector were postponed during the pandemic. Moreover, the COVID-19 pandemic had a negative impact on the ongoing clinical trials for skeletal dysplasia, which is anticipated to hinder the growth of the global skeletal dysplasia market during the projected period as a result of the delayed clinical trials of products.
In addition, consumers focused only on highly essential products such as foods & beverages. However, with relaxation in lockdowns and decline in COVID-19 cases in 2022, companies re-started their processes to meet the demand of products. Owing to the introduction of various COVID-19 vaccinations, people can readily reach out to hospital and pharmacy due to which surge in the demand for skeletal dysplasia product for the treatment of skeletal dysplasia is increased. Thus, such development is anticipated to bring stabilization and drive the opportunities of the skeletal dysplasia market forecast.
Segmental Overview
The skeletal dysplasia market is segmented into type, treatment, distribution channel and region. By type, the market is categorized into morquio A syndrome, X-linked hypophosphatemia, hypophosphatasia and others. On the basis of treatment, the market is segregated into enzyme replacement therapy, human monoclonal antibody and others. On the basis of distribution channel, the market is divided into hospital pharmacies, drug stores and specialty pharmacies and online providers. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
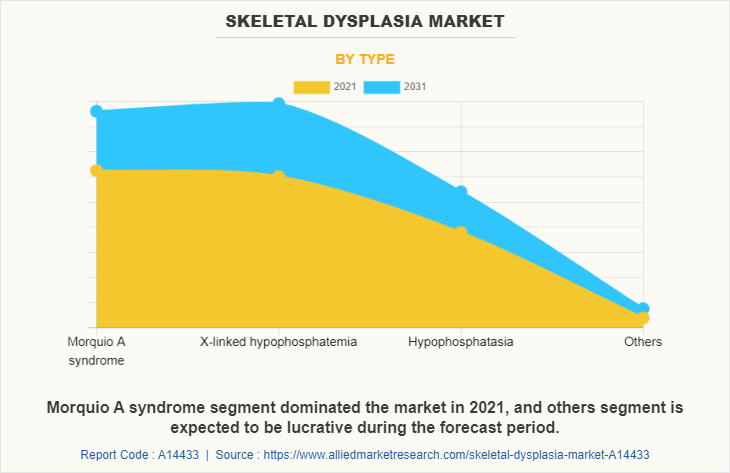
By Type
Based on type, the market is segmented into morquio A syndrome, X-linked hypophosphatemia, hypophosphatasia and others. The morquio A syndrome segment dominated the skeletal dysplasia market size in 2021, and is expected to remain dominant throughout the forecast period, owing to the increasing incidence of morquio A syndrome. On the other side, others segment is projected to exhibit the fastest market growth during the forecast period, owing to increase in clinical trials forof achondroplasia and osteogenesis imperfecta.
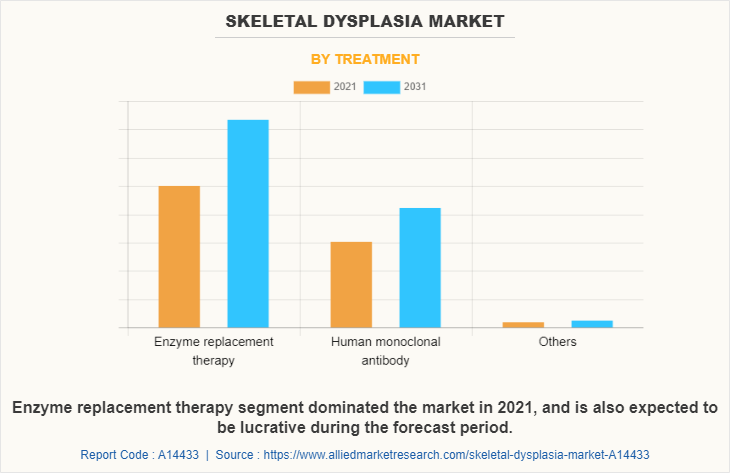
By Treatment
The skeletal dysplasia market is segregated into enzyme replacement therapy, human monoclonal antibody and others. The enzyme replacement therapy segment dominated the skeletal dysplasia market size in 2021 and is anticipated to continue this trend during the forecast period, owing to rise in the prevalence of rare diseases has significantly increased the use of enzyme replacement therapy which boost the growth of the market. In addition, initiatives taken by various government and private organizations to spread awareness about enzyme replacement therapy drives the market.
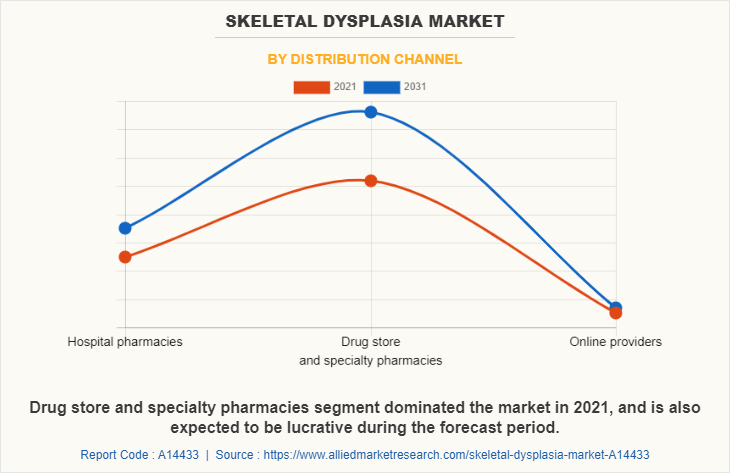
By Distribution Channel
The skeletal dysplasia market is segregated into hospital pharmacies, drug stores and specialty pharmacies and online providers. The drug stores and specialty pharmacies segment dominated the skeletal dysplasia market share in 2021 and is anticipated to continue this trend during the forecast period. This is attributed to availability of high cost, high touch medication therapy for patients with complex disease.
By Region
The skeletal dysplasia market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for a major share of the skeletal dysplasia market in 2021 and is expected to maintain its dominance during the forecast period.
Key factors drive the growth of the market are substantial presence of major pharmaceutical and biopharmaceutical companies in the North American region. Additionally, it is anticipated that rising demand for preventative healthcare further boost the market growth. Moreover, increasing government and private sector efforts to promote healthy lifestyles are anticipated to fuel market expansion in the region during the forecast period. In addition, presence of well-established healthcare infrastructure, high purchasing power, and rise in adoption rate of skeletal dysplasia products are expected to drive the market growth. Moreover, increase in prevalence of eye disease in the region as well as rise in awareness about the disease prevention among people lead to rise in demand for the skeletal dysplasia treatment, and further boost the growth of the market.
Asia-Pacific expected to grow at the highest rate during the forecast period. The market growth in this region is attributable to presence of pharmaceutical companies in the region as well as growth in the purchasing power of populated countries, such as China and India. Moreover, rise in pediatric population and increase in prevalence of skeletal dysplasia including achondroplasia, osteogenesis imperfecta, thanatophoric dysplasia, hypochondroplasia and others drive the growth of the market. Furthermore, the Asia-Pacific region exhibits the high population base, presence of unmet medical needs, and increase in disposable incomes of people in the region. Moreover, rise in medical tourism and developing healthcare infrastructure make Asia-Pacific a lucrative market for skeletal dysplasia treatment. Asia-Pacific offers profitable opportunities for key players operating in the skeletal dysplasia market, thereby registering the fastest growth rate during the forecast period, owing to the growing infrastructure of industries, rising disposable incomes, as well as well-established presence of domestic companies in the region. In addition, rise in contract manufacturing organizations within the region provides great opportunity for new entrants in this region.
Competition Analysis
Competitive analysis and profiles of the major players in the healthcare assistive robot, such as Amgen Inc., Ascendis pharma a/s, AstraZeneca plc., Biomarin Pharmaceuticals Inc., Cipla Ltd, Eli Lilly and Company, F. Hoffmann-La Roche AG, Merck KGaA, Pfizer, Inc., and Teva Pharmaceutical Industries Ltd. Major players have adopted product approval and acquisition as key developmental strategies to improve the product portfolio of the skeletal dysplasia market.
Some Examples Of Acquisition In The Market
In May 2019, Pfizer Inc. Was announced the successful completion of its acquisition of the privately held clinical-stage biotechnology company Therachon Holding AG. Under the terms of the transaction, Pfizer acquired Therachon for $340 million with an additional $470 million in additional payments contingent on the achievement of key milestones in the development and commercialization of TA-46. TA-46 is an investigational medicine for the treatment of achondroplasia, a genetic condition and the most common form of short-limb dwarfism.
Some Examples Of Product Approval In The Market
In November 2021, BioMarin Pharmaceutical Inc. was announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval to VOXZOGO (vosoritide) for Injection, indicated to increase linear growth in pediatric patients with achondroplasia five years of age and older with open epiphyses (growth plates).
In January 2022, Ascendis Pharma A/S was announced that the European Commission (EC) has granted marketing authorization for Lonapegsomatropin Ascendis Pharma (developed under the name TransCon hGH) as a once-weekly subcutaneous injection for the treatment of children and adolescents ages 3 to 18 years with growth failure due to insufficient secretion of endogenous growth hormone (also known as growth hormone deficiency, or GHD). TransCon hGH is a prodrug of somatropin that provides sustained release of unmodified somatropin (hGH) at predictable therapeutic levels in the body.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the skeletal dysplasia market analysis from 2021 to 2031 to identify the prevailing skeletal dysplasia industry opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the skeletal dysplasia market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global skeletal dysplasia market trends, key players, market segments, application areas, and market growth strategies.
Skeletal Dysplasia Market Report Highlights
| Aspects | Details |
| Market Size By 2031 | USD 2.4 billion |
| Growth Rate | CAGR of 3.7% |
| Forecast period | 2021 - 2031 |
| Report Pages | 355 |
| By Type |
|
| By Treatment |
|
| By Distribution Channel |
|
| By Region |
|
| Key Market Players | Teva Pharmaceutical Industries Ltd., AstraZeneca plc, Eli Lilly and Company, Ascendis pharma a/s, Biomarin Pharmaceuticals Inc., Cipla Ltd, F. Hoffmann-La Roche AG, Merck KGaA, Pfizer, Inc., Amgen Inc. |
Analyst Review
This section provides opinions of top level CXOs in the skeletal dysplasia market. According to insights of CXOs, skeletal dysplasia treatment is gaining high traction in the market, owing to increase in prevalence of achondroplasia, osteogenesis imperfecta and others, rise in R&D investments in drug discovery & development, and increase in awareness skeletal dysplasia treatments.
Furthermore, key players in the market are focusing on adopting strategies to increase accessibility and utilization of skeletal dysplasia treatment products in developing economies. Moreover, the market gains interest of healthcare companies, owing to its unmet demands in developing economies such as India and China where the population is growing rapidly which further propel the market growth.
North America is expected to witness highest growth, in terms of revenue, owing to, robust healthcare infrastructure, presence of key players, and rise in healthcare expenditure. However, Asia-Pacific is anticipated to witness notable growth, owing to rise in pediatric population, unmet medical demands and increase in public–private investments in the healthcare sector.
Increase in number of cases of skeletal dysplasia and a large number of products in the pipeline for treatment of various type of skeletal dysplasia are the upcoming trends.
Enzyme replacement therapy is the leading treatment for skeletal dysplasia.
North America is the largest regional market for skeletal dysplasia.
The global skeletal dysplasia market accounted for $1,638.10 million in 2021, and is expected to reach $2,360.51 million by 2031, registering a CAGR of 3.7% from 2022 to 2031.
The report provides a comprehensive analysis of the key players that operate in the global skeletal dysplasia market. The key companies profiled in the report include Amgen Inc., Ascendis Pharma a/s, AstraZeneca plc., Biomarin Pharmaceuticals Inc., Cipla Ltd, Eli Lilly and Company, F. Hoffmann-La Roche AG, Merck KGaA, Pfizer, Inc., and Teva Pharmaceutical Industries Ltd.
2021 is the base year of skeletal dysplasia market.
2022 to 2031 is the forecast period of skeletal dysplasia market.
Loading Table Of Content...


