TAVR Embolic Protection Market Research, 2032
The global tavr embolic protection market size was valued at $108.8 million in 2022, and is projected to reach $405.4 million by 2032, growing at a CAGR of 14% from 2023 to 2032. The growth of the TAVR embolic protection market size is significantly driven by the increasing prevalence of aortic stenosis and the surge in the geriatric population. Aortic stenosis (AS), a condition characterized by the narrowing of the aortic valve in the heart, has become more prevalent as the global population ages. For instance, according to the European Society of Cardiology report in 2020, stated that prevalence of AS is 12.4%, and a prevalence of severe AS in those aged 75 years and older is 3.4%.
The elderly are more susceptible to this ailment, which has led to a rising demand for TAVR procedures. As a minimally invasive alternative to open-heart surgery, TAVR is increasingly preferred among elderly patients due to its reduced recovery times and lower risks. This demographic shift, marked by a growing elderly population, creates a substantial patient pool for TAVR, consequently fueling the demand for embolic protection devices. As these factors persist, the TAVR embolic protection market is expected to continue its growth trajectory in the coming years.
Key Takeaways
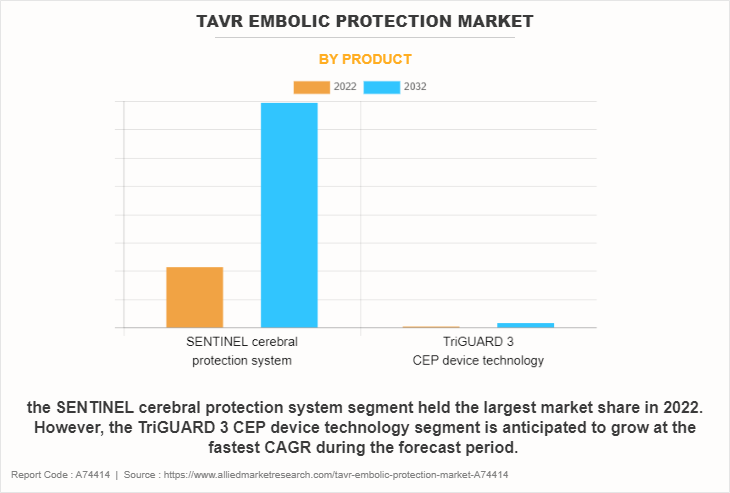
- The SENTINEL Cerebral Protection System segment dominated the market in terms of revenue in 2022. However, the TriGUARD 3 CEP Device Technology segment is anticipated to grow at the fastest CAGR during the forecast period.
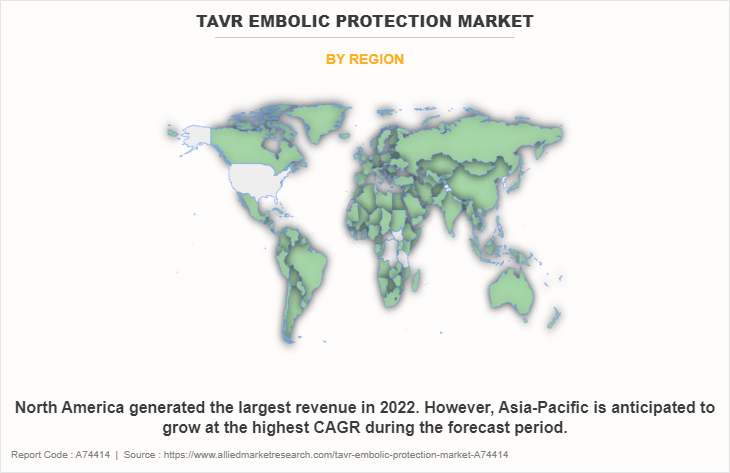
- The North America region dominated the market in terms of revenue in 2022.
TAVR, also known as transcatheter aortic valve implantation (TAVI), is a minimally invasive procedure designed to treat aortic valve stenosis, a condition characterized by the narrowing of the aortic valve in the heart. This narrowing reduces blood flow from the heart to the rest of the body, leading to symptoms such as chest pain, shortness of breath, and fatigue. Traditional treatment for aortic stenosis involves open-heart surgery to replace the damaged valve. However, TAVR has emerged as a revolutionary alternative. However, during this process, small debris or embolic material can be dislodged and enter the bloodstream, potentially leading to stroke or other complications. This is where embolic protection devices come into play.
Embolic protection devices are specialized filters or systems that are placed within the blood vessels to capture and remove embolic material released during the TAVR procedure. They act as a safety net, preventing these particles from traveling to critical organs like the brain.
Market Dynamics
Advancements in healthcare infrastructure, particularly in developing economies, play a significant role in the TAVR Embolic protection market growth by improving access to TAVR embolic protection market and increase in awareness about their benefits. In addition, continuous investments in R&D activities by pharmaceutical companies lead to the introduction of an innovative TAVR embolic protection market with enhanced efficacy and safety profiles which further drive growth of TAVR embolic protection industry.
Furthermore, the presence of robust reimbursement policies is playing a pivotal role in driving the growth of the TAVR embolic protection market. Reimbursement policies ensure that healthcare providers are financially incentivized to offer TAVR procedures with embolic protection devices to their patients. These policies often cover a significant portion of the costs associated with these procedures, making them more accessible and affordable for patients.
For healthcare facilities and providers, this translates into a reliable source of revenue, as they can confidently offer TAVR with embolic protection knowing that they will be reimbursed for their services. This financial security encourages the adoption of TAVR procedures and embolic protection devices, contributing to TAVR embolic protection industry growth.
Moreover, reimbursement policies provide a sense of assurance for patients, alleviating concerns about the financial burden of these potentially life-saving procedures. Patients are more likely to opt for treatments covered by insurance, which, in turn, boosts the demand for TAVR embolic protection devices.
However, there are certain factors that act as impediments to the growth of the TAVR embolic protection market. These include the complexities involved in the procedures and the elevated costs associated with cerebral embolic protection devices. The deployment of such devices often demands specialized training for healthcare practitioners, which can pose a challenge to their widespread use. Moreover, the high cost of cerebral embolic protection devices can be a significant deterrent, particularly in regions where healthcare budgets are constrained. Therefore, the combination of these factors can hinder the broader adoption of these devices and, subsequently, the expansion of the market.
The need for medical devices remains relatively constant, even in economic downturns, as they are essential for treating severe health conditions. Additionally, the increasing aging population and the prevalence of cardiovascular diseases, including aortic stenosis, along with the rising adoption of minimally invasive TAVR procedures, continue to bolster the consistent demand for medical devices such as cerebral embolic protection devices.
Furthermore, substantial investments and the unwavering commitment of major players to develop advanced cerebral embolic protection devices have demonstrated resilience and potential for market expansion. These investments play a critical role in the creation of more sophisticated and efficient devices that enhance patient safety during TAVR procedures. Additionally, ongoing clinical trials focused on innovative embolic protection technologies underscore a dedication to improving patient outcomes. As healthcare providers seek cost-effective and streamlined solutions, the TAVR embolic protection market remains well-positioned for sustained long-term growth, driven by innovation, research, and the pursuit of safer medical interventions.
Segmental Overview
The TAVR embolic protection market is segmented into product type and region. By product type, the market is categorized into SENTINEL Cerebral Protection System and TriGUARD 3 CEP Device Technology. Region-wise, the market is analyzed across North America (the U.S. and Canada), Europe (Germany, France, the UK, Italy and rest of Europe), Asia-Pacific (Japan, China, Australia, India and rest of Asia-Pacific), and LAMEA (Latin America and Middle East & Africa).
By Product Type
The TAVR embolic protection market is segmented into product type and region. By product type, the market is categorized into SENTINEL Cerebral Protection System and TriGUARD 3 CEP Device Technology. The SENTINEL Cerebral Protection System segment attained the TAVR embolic protection market share in 2022 and is expected to remain dominant during the forecast period. The SENTINEL system is renowned as a trailblazing and well-established brand within the TAVR embolic protection market, known for its strong track record of safety and effectiveness, which has earned it the trust of healthcare providers. Moreover, it is the only FDA and CE approved device in the market.
However, the TriGUARD 3 CEP Device Technology segment is projected to register the fastest CAGR during the forecast period, owing to its cutting-edge innovative technology, clinical efficacy, expanded indications, and the preference among physicians.
By Region
The TAVR embolic protection market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for the largest TAVR embolic protection market share in terms of revenue in 2022, owing to its well-developed healthcare industry, rise in cardiovascular diseases including aortic stenosis, and increase in number of key players offering TAVR embolic protection devices. The U.S. serves to be the prime market in North America, owing to technological advancements in TAVR embolic protection devices and presence of aged population are anticipated to drive the growth of the market. In addition, the surge in demand for minimally invasive procedures such as TAVR and the availability of skilled professionals and advanced embolic protection devices technologies has made it possible to perform more complex procedures with higher success rates, leading to increased demand for TAVR embolic protection devices in North America.
However, on the other hand, Asia-Pacific is expected to witness the highest growth during the TAVR Embolic protection market forecast period owing to the presence of a high population base, surge in geriatric population, and high growth potential in emerging economies. In addition, the growing demand for minimally invasive procedures are further driving the market growth. Similarly, increase in the number of initiatives and significant investments from governments for overall development of healthcare system propel the growth of the market in Asia-Pacific region.
Moreover, a rise in research activities as well as the well-established presence of domestic companies in the region are expected to provide notable opportunities for market growth. In addition, a rise in contract manufacturing organizations within the region provides is expected to open new avenues for market growth.
Competition Analysis
Competitive analysis and profiles of the major players in the TAVR embolic protection market such as Major players that operate in the market include Boston Scientific Corporation, Edward Lifesciences Corporation, Filterlex Medical Ltd., Innovative Cardiovascular Solutions, LLC., Protembis GmbH, Transverse Medical Inc., Emboline, Inc., Cardioptimus, EnCompass Technologies, Inc., Venus MedTech HangZhou Inc. Key players have adopted strategies such as product approval, funding, and clinical trials to enhance their product portfolio.
Recent Clinical Trials in the TAVR Embolic Protection Market
- In May 2022, Filterlex Medical Ltd., a cardiovascular medical device startup, announced results from a first-in-human (FIH) study demonstrating the safety, feasibility, and performance of the CAPTIS device. The trial was a prospective, single-arm study involving 20 patients who underwent a successful Transcatheter Aortic Valve Replacement (TAVR) procedure while using the CAPTIS embolic protection device.
Recent Funding in the TAVR Embolic Protection Market
- In May 2021, Emboline, Inc., a privately held medical device company focused on reducing stroke and other damage caused by embolic debris released during transcatheter heart procedures, today announced the closing of its Series D funding of over$55 million. This investment will support a planned US-based pivotal trial for FDA approval as well as investigational studies for new indications, and manufacturing and commercial operations for the Emboliner.
- In December 2021, Filterlex Medical, a cardiovascular medical device startup, announced that it has been selected to receive $7.34 Million equity investment from the European Innovation Council Accelerator (EIC). The EIC equity investment will be a part of Filterlex’s next round of financing. This combined funding will go towards clinical program expansion, regulatory approvals, and commercialization of the CAPTIS full-body embolic protection system to help transform left-heart procedures.
Recent Product Approval in TAVR Embolic Protection Market
- In May 2020, Keystone Heart Ltd., a Venus Medtech Company, received European CE Mark for the TriGUARD 3 Cerebral Embolic Protection (CEP) Device. The device is designed to minimize the risk of cerebral damage by deflecting embolic debris away from cerebral circulation during Transcatheter Aortic Valve Implantation (TAVI) and other transcatheter heart procedures.
- In September 2023, EnCompass Technologies, Inc., a privately held medical device company focused on protecting patients from brain injury during cardiovascular procedures, announced that the U.S. Food and Drug Administration (FDA) officially granted conditional Investigational Device Exemption (IDE) approval for its F2 cerebral embolic protection system.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the tavr embolic protection market analysis from 2022 to 2032 to identify the prevailing tavr embolic protection market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the tavr embolic protection market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global tavr embolic protection market trends, key players, market segments, application areas, and market growth strategies.
TAVR Embolic Protection Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 405.4 million |
| Growth Rate | CAGR of 14% |
| Forecast period | 2022 - 2032 |
| Report Pages | 140 |
| By Product |
|
| By Region |
|
| Key Market Players | EnCompass Technologies, Inc., Edward Lifesciences Corporation, Emboline, Inc., Boston Scientific Corporation, Innovative Cardiovascular Solutions, LLC., Transverse Medical Inc., Cardioptimus, Venus MedTech HangZhou Inc., Protembis GmbH, Filterlex Medical Ltd. |
Analyst Review
The demand for efficient and high-quality devices for prevention of complications associated with the TAVR procedures such as strokes and others are expected to offer profitable opportunities for the expansion of the market. In addition, a rise in awareness among healthcare professionals about the use of cerebral embolic protection devices in the TAVR procedure is anticipated to boost the market growth.
The increase in incidence of cardiovascular diseases including aortic stenosis in the population of developed as well as developing regions has largely contributed toward the revenue growth in 2022 and is expected to maintain this trend during the forecast period. Moreover, market players have invested in the development of technologically advanced devices with improved efficacy and quality, which is expected to drive the growth of the market.
Moreover, the TAVR embolic protection market has become increasingly competitive, with a growing number of companies developing advanced technologies. Furthermore, North America accounted for the largest share in terms of revenue in 2022, owing to advancement in technology, increase in number of TAVR surgical procedures carried out, and upsurge in number of key players investment in the R&D. However, Asia-Pacific is anticipated to witness notable growth, owing to rise in aging population, growing adoption of minimally invasive procedures such as TAVR, and surge in prevalence of strokes cases.
TAVR embolic protection devices are specialized medical tools used during Transcatheter Aortic Valve Replacement (TAVR) procedures. They are designed to capture and contain embolic debris, such as blood clots or particles, preventing them from traveling to the brain and causing complications such as strokes.
Top companies such as Boston Scientific Corporation, Venus MedTech HangZhou Inc., Filterlex Medical Ltd. and EnCompass Technologies, Inc. held a high market position in 2022. These key players held a high market postion owing to the strong geographical foothold in North America, Europe, Asia-Pacific, and LAMEA.
The major factor that fuels the growth of the TAVR embolic protection market are reduced mortality rate ,technological advancements in TAVR embolic protection devices and growing adoption of minimally invasive procedures drive the growth of the global TAVR embolic protection market .
The SENTINEL Cerebral Protection System segment is the most influencing segment in TAVR embolic protection market.This is attributed to Its reputation for safety and efficacy has garnered trust among healthcare providers. Moreover, it is the only FDA and CE approved device in the market.
The market value of TAVR embolic protection market in 2032 is $ 405,440.67 thousand
The forecast period for TAVR embolic protection market is 2023 to 2032
The base year is 2022 in TAVR embolic protection market .
The total market value of TAVR embolic protection market is $108,782.53 thousand in 2022.
Loading Table Of Content...
Loading Research Methodology...



