Global Therapeutic Vaccines Market Research, 2033
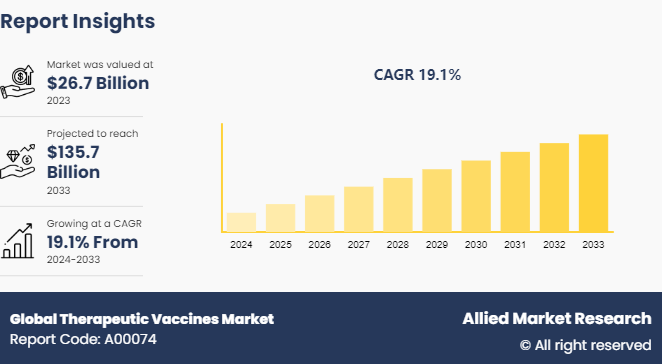
The global global therapeutic vaccines market size was valued at $26.7 billion in 2023 and is projected to reach $135.7 billion by 2033, growing at a CAGR of 19.1% from 2024 to 2033.
Market Introduction and Definition
A therapeutic vaccination is one that is given after a sickness or infection has already been developed. A therapeutic vaccine activates a patient's immune system to fight against an infection. A therapeutic vaccine varies from a prophylactic vaccination. Prophylactic vaccines are given to people as a preventative strategy to avert infection or disease, whereas therapeutic vaccines are given after the person has been impacted by the sickness or infection. A therapeutic vaccination treats an existing infection in the body rather than immunizing it against future diseases and infections. Therapeutic vaccinations are mostly used against viral illnesses. Patients with chronic viral infections are provided therapeutic vaccinations because their immune system is unable to produce enough efficient antibodies.
Key Takeaways
- The therapeutic vaccines market growth study covers 20 countries. The research includes a segment analysis of each country in terms of value ($billion) for the projected period from 2024 to 2033.
- More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major energy storage system industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrates high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach intends to provide a balanced view of global markets and assist stakeholders in making informed decisions in order to achieve their most ambitious growth objectives.
Key Market Dynamics
Therapeutic vaccines represent a novel approach in immunotherapy, designed to harness the body's immune system not merely for preventing diseases, but for actively treating existing conditions and slowing their progression. Unlike traditional vaccines, which primarily aim to preempt infections by inducing the production of antibodies against specific pathogens, therapeutic vaccines stimulate the immune system to recognize and combat diseased cells, such as cancer cells, or to manage chronic infections like HIV. By targeting these abnormal cells, therapeutic vaccines can help control the disease, reduce symptoms, and improve the quality of life for patients. These factors are anticptaed to boost the therapeutic vaccines industry growth.
The R&D process for therapeutic vaccines is notably expensive and time-consuming, posing a significant barrier to market entry and expansion. Developing these vaccines involves extensive preclinical studies and clinical trials to ensure safety and efficacy, which require substantial financial investments. In addition, the sophisticated technology and specialized expertise needed to create effective therapeutic vaccines further drive-up costs. This financial burden can deter smaller companies from entering the market and slow the progress of even well-established firms, ultimately impacting the pace at which new therapeutic vaccines become available to patients.
The global therapeutic vaccines market size is experiencing significant expansion, driven by increased government funding for vaccine development, increased investments by major therapeutic vaccines industry players, technological advancements, and initiatives from NGOs. The rising prevalence of diseases such as cancer, HIV, neurological diseases, autoimmune diseases, and infectious diseases is leading to an increase in demand for therapeutic vaccines. For instance, as per World Health Organization, in 2022, there were 20 million new cancer diagnoses and 9.7 million fatalities. The expected number of people who survived 5 years after a cancer diagnosis was 53.5 million. This growing incidence of severe diseases highlights the urgent need for effective therapeutic vaccines, thereby propelling therapeutic vaccines market opportunity and increasing the adoption of these innovative treatments.
Global Vaccines Market Overview
Vaccines are an essential tool for preventing diseases and reducing the burden these diseases place on both governments and families. Vaccines have saved millions of people across the globe from various illnesses. By getting vaccinated, individuals are protected against serious illness and, sometimes, death from vaccine-preventable diseases. Vaccination also helps prevent the spread of these diseases among family members, friends, colleagues, classmates, and the wider community.
According to a report published by the WHO in 2022, the vaccine market is highly concentrated, with approximately 80 to 85% of the market share captured by the top 10 manufacturers: Pfizer, Moderna, Sanofi, China National Biotec Group (CNBG) , AstraZeneca, Serum Institute of India Pvt. Ltd., BioNTech SE, Merck & Co., Inc., Johnson & Johnson Services, Inc., and GSK plc.
Global Vaccines Market Overview
Market Share by Value in 2022 | 2022 |
Pfizer | 36% |
Moderna | 15% |
Sanofi | 6% |
China National Biotec Group (CNBG) | 3% |
AstraZeneca | 2% |
Serum Institute of India Pvt. Ltd. | 2% |
BioNTech SE | 3% |
Merck & Co., Inc. | 9% |
Johnson & Johnson Services, Inc. | 2% |
GSK plc | 8% |
Others | 14% |
Market Segmentation
The therapeutic vaccines market share is segmented into product, technology, and region. On the basis of product, the market is divided into autoimmune disease vaccines, neurological disease vaccines, cancer vaccines, infectious disease vaccines, and others. As per technology, the market is classified into allogeneic vaccine and autologous vaccine. Region wise, the therapeutic vaccines market share is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional Market Outlook
The North America therapeutic vaccines market forecast is experiencing robust growth, driven by the presence of a developed healthcare infrastructure, a supportive ecosystem, and high expenditure on healthcare. In addition, the presence of some of the major vaccine manufacturers in the region further drives market expansion. According to a report published by Radar Healthcare in June 2022, the U.S. spends 0.81% of its GDP on healthcare expenditure.
- In June 2022, GSK announced that the U.S. FDA had approved Priorix for the prevention of measles, mumps, and rubella in individuals aged 12 months and older.
- In April 2022, a team at King's College London purportedly discovered the world's first cure for heart attacks utilizing mRNA technology similar to COVID-19 vaccinations.
- In March 2022, the National Institute of Allergy and Infectious Diseases (NIAID) , a branch of the National Institutes of Health (NIH) , launched a Phase 1 clinical trial to assess three experimental HIV vaccines utilizing messenger RNA (mRNA) technology. This trial, named HVTN 302, was sponsored by NIAID and carried out by the HIV Vaccine Trials Network (HVTN) , which is funded by NIAID and located at the Fred Hutchinson Cancer Research Center in Seattle.
Competitive Landscape
The major players operating in the therapeutic vaccines market include Agenus Inc. Argos Therapeutic Inc., Celldex Therapeutic Inc., Dendreon Pharmaceuticals LLC., GSK plc, Merck & Co. Inc., Novartis AG, Pfizer, Inc., Phio Pharmaceuticals Corp., INOVIO Pharmaceuticals, AstraZeneca, and BioNTech SE.
Other players in the therapeutic vaccines market include Moderna, Sanofi, China National Biotec Group (CNBG) , Serum Institute of India Pvt. Ltd., and Johnson & Johnson Services, Inc.
Recent Key Strategies and Developments
- On March 27, 2024, Moderna, Inc. announced at its fifth Vaccines Day event various clinical and program updates that highlighted the progress and acceleration of its mRNA pipeline. The updates included data readouts from the company's respiratory, latent, and other vaccine portfolios, along with commercial, manufacturing, and financial announcements related to its vaccines business.
- In March 2024, AstraZeneca revealed that it had reached an agreement to purchase Amolyt Pharma, a biotechnology company in the clinical stages of developing innovative treatments for rare endocrine disorders.
- In October 2023, Pfizer Inc. announced that the U.S. Food and Drug Administration approved PENBRAYA, a vaccine covering meningococcal groups A, B, C, W, and Y. This vaccine became the first and only pentavalent vaccine providing protection against the most common serogroups causing meningococcal disease in adolescents and young adults aged 10 to 25 years.
Industry Trends
- In March 2024, at the EUROGIN 2024 HPV Congress, Merck, also known as MSD outside the U.S. and Canada, announced that it had initiated clinical development of a new investigational multi-valent HPV vaccine aimed at offering broader protection against multiple HPV types. In addition, the company revealed plans to conduct clinical trials involving both females and males to assess the efficacy and safety of a single-dose regimen of GARDASIL 9 (Human Papillomavirus 9-valent, recombinant) compared to the approved three-dose regimen.
- In December 2023, AstraZeneca finalized an agreement to acquire Icosavax, Inc., a US-based clinical-stage biopharmaceutical firm dedicated to creating distinctive, high-potential vaccines utilizing an innovative protein virus-like particle (VLP) platform.
Key Sources Referred
- Eastgate Dental Excellence.
- WHO
- American Association of Orthodontists
- Align Technology, Inc.
- Envista
- Cleveland Clinic
- World Federation of Orthodontists
- International Association for Orthodontics
- MJH Life Sciences
- Oral Health Foundation
- WebMD LLC
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the global therapeutic vaccines market analysis from 2023 to 2033 to identify the prevailing global therapeutic vaccines market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the global therapeutic vaccines market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
Apart from the points mentioned above, the report includes the analysis of the regional as well as global global therapeutic vaccines market trends, key players, market segments, application areas, and market growth strategies.
Global Therapeutic Vaccines Market Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 135.7 Billion |
| Growth Rate | CAGR of 19.1% |
| Forecast period | 2024 - 2033 |
| Report Pages | 300 |
| By Product |
|
| By Technology |
|
| By Region |
|
| Key Market Players | Dendreon Pharmaceuticals LLC, Novartis AG, Merck & Co. Inc., Pfizer, Inc., BioNTech SE, AstraZeneca, Celldex Therapeutics Inc., Agenus Inc., GSK plc., Argos Therapeutics, Inc. |
Upcoming trends in the global therapeutic vaccines market include advancements in personalized medicine, increased focus on cancer vaccines, and the development of novel vaccine delivery systems.
The leading application of the Global Therapeutic Vaccines Market is cancer treatment.
Asia-Pacific is the largest regional market for global therapeutic vaccines market.
The therapeutic vaccines market was valued at $26.7 billion in 2023 and is estimated to reach $135.7 billion by 2033, exhibiting a CAGR of 19.1% from 2024 to 2033.
The major players operating in the therapeutic vaccines market include Agenus Inc. Argos Therapeutic Inc., Celldex Therapeutic Inc., Dendreon Pharmaceuticals LLC., GSK plc, Merck & Co. Inc., Novartis AG, Pfizer, Inc., Phio Pharmaceuticals Corp., INOVIO Pharmaceuticals, AstraZeneca, and BioNTech SE.
Loading Table Of Content...


