U.S. Vaccines Market Research, 2033
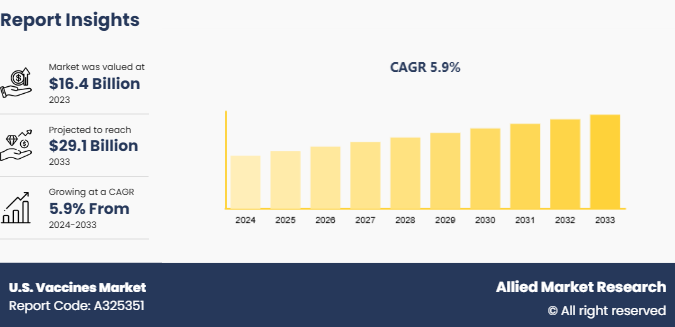
The U.S. vaccines market size was valued at $16.4 billion in 2023, and is projected to reach $29.1 billion by 2033, growing at a CAGR of 5.9% from 2024 to 2033. The U.S. vaccines market is driven by increasing vaccination programs and government initiatives aimed at preventing infectious diseases. Rising public awareness and the demand for preventive healthcare further support the U.S. vaccines market growth.
A vaccine is a biological substance designed to provide immunity against a specific disease. It typically contains weakened or inactivated forms of pathogens, such as viruses or bacteria, or parts of them, such as proteins or genetic material. Vaccination plays a crucial role in public health by reducing the spread of infectious diseases.
Key Market Dynamics
The U.S. vaccines market growth is driven by factors such as rise in public awareness about immunization, increase ingovernment initiatives promoting vaccination programs, and rise in prevalence of infectious diseases. The development of new vaccines targeting emerging diseases, coupled with advancements in biotechnology and vaccine production technologies, has significantly contributed to U.S. Vaccines market share. For instance, in June 2024, Merck, known as MSD outside of the U.S. and Canada, announced that the U.S. Food and Drug Administration (FDA) has approved CAPVAXIVE (Pneumococcal 21-valent Conjugate Vaccine) ?for prevention of invasive pneumococcal disease and pneumococcal pneumonia in adults.
However, the market faces several restraints such as high cost of vaccine development and production, which often requires extensive research, clinical trials, and regulatory approval processes. This can lead to high prices for end consumers, limiting access, especially among low-income populations. In addition, vaccine hesitancy, driven by misinformation and safety concerns, remains a barrier to achieving optimal immunization coverage. Despite widespread efforts to educate the public about vaccine safety, skepticism about vaccine efficacy continues to affect market penetration, particularly in certain regions of the U.S.
On the other hand, government policies aimed at improving healthcare infrastructure and expanding access to vaccines offer U.S. vaccines market opportunity. Programs such as the Vaccines for Children (VFC) program, which provides free vaccines to uninsured children, and initiatives to enhance adult immunization rates are expected to drive growth during U.S. vaccines market forecast period. Moreover, introduction of new vaccine delivery methods, such as nasal sprays, patches, and oral formulations, presents an opportunity to increase vaccine consumption by improving convenience and accessibility. Collaborations between public health organizations, vaccine manufacturers, and global health bodies are also likely to foster growth, ensuring the U.S. vaccines market size remains at the forefront of global immunization efforts.
Market Segmentation
The U.S. vaccines market share is segmented on the basis of technology type, indication, and end user. On the basis of technology type, the market is categorized into recombinant and conjugate vaccines, live attenuated vaccines, inactivated vaccines, toxoid vaccines, and others. On the basis of indication, the market is classified into pneumococcal disease, influenza, human papilloma virus, meningococcal disease, rotavirus, varicella, measles, mumps, and rubella, diphtheria, pertussis, and tetanus (DTP) , polio, hepatitis, and other indications. On the basis of end user, the market segmented into pediatric, adults, and travelers.
Industry Trends
- In September 2024, the Food and Drug Administration (FDA) approved the FluMist for self- or caregiver-administration, enhancing accessibility for individuals aged 2 to 49. This approval allows for greater convenience in receiving vaccinations, as it can now be administered at home after a screening process. FluMist is approved for the prevention of influenza disease caused by influenza virus subtypes A and B in individuals 2 through 49 years of age.
- In July 2024, the Centers for Disease Control and Prevention (CDC) continued to recommend routine vaccination against rotavirus for infants in the U.S. There are two approved vaccines, namely, RotaTeq (3 doses) and Rotarix (2 doses, which are administered orally, with the first dose recommended before 15 weeks of age and all doses completed by 8 months old. The CDC emphasizes that these vaccines are both safe and effective, significantly reducing hospitalizations due to rotavirus, which previously caused about 40, 000 to 50, 000 hospital visits annually among U.S. infants.
Competitive Landscape
The key players operating in the U.S. vaccines industry are Bavarian Nordic, Merck & Co., Inc., GSK plc., Sanofi, Pfizer, Emergent Biosolutions, CSL, Moderna, Inc., Dynavax Technologies, and Novavax. These players have adopted various developmental strategies to stay competitive in the market. For instance, in April 2024, Bavarian Nordic A/S announced that JYNNEOS, the only FDA-approved mpox vaccine, is now commercially available in the U.S., marking a significant expansion for access to JYNNEOS by establishing additional pathways for vaccine procurement, distribution, and reimbursement by both public and private payers. In addition, in October 2023, Pfizer Inc. received the U.S. Food and Drug Administration (FDA) approval for PENBRAYA (meningococcal groups A, B, C, W and Y vaccine) , the first and only pentavalent vaccine that provides coverage against the most common serogroups causing meningococcal disease in adolescents and young adults 10 through 25 years of age.
Key Benefits for Stakeholders
- Enable informed decision-making process and offer based on current market situation and estimated future trends.
- Analyze the key strategies adopted by major market players in U.S. vaccines industry.
- Assess and rank the top factors that are expected to affect the growth of U.S. vaccines market trends.
- Top Player positioning provides a clear understanding of the present position of market players.
- Detailed U.S. vaccines market analysis and segmentation assists to determine the prevailing market opportunities.
- Identify key investment pockets for various offerings in the market.
U.S. Vaccines Market Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 29.1 Billion |
| Growth Rate | CAGR of 5.9% |
| Forecast period | 2024 - 2033 |
| Report Pages | 90 |
| By Technology Type |
|
| By Indication |
|
| By End User |
|
| Key Market Players | Bavarian Nordic, GSK plc., Sanofi, Novavax, Inc., Merck & Co., Inc., Moderna, Inc., CSL, Emergent Biosolutions, Pfizer, Dynavax Technologies |
The U.S. Vaccines Market is projected to grow at a CAGR of 5.9% from 2024 to 2033
Bavarian Nordic, Merck & Co., Inc., GlaxoSmithKline plc., Sanofi, Pfizer, Emergent Biosolutions, CSL, Moderna, Inc., Dynavax Technologies, Novavax, Inc. are the leading players in U.S. Vaccines Market.
1. Enable informed decision-making process and offer market analysis based on current market situation and estimated future trends. 2. Analyze the key strategies adopted by major market players in U.S. vaccines market. 3. Assess and rank the top factors that are expected to affect the growth of U.S. vaccines market. 4. Top Player positioning provides a clear understanding of the present position of market players. 5. Detailed analysis of the U.S. vaccines market segmentation assists to determine
For analysis, the U.S. Vaccines market is segmented into technology type, indication, and end user.
Loading Table Of Content...



