Viral Clearance Market Research, 2031
The global viral clearance market size was $425.9 million in 2021, and is projected to reach $977.8 million by 2031, growing at a CAGR of 8.6% from 2022 to 2031. Viral clearance is an essential process in development of monoclonal antibodies, recombinant proteins and glycoproteins, tissue and blood-derived products, and medical devices. This is attributed to the fact that these products are at risk of contamination by bacteria and fungi, chemical impurities, and viruses. The viral clearance and evaluation are the significant steps in product safety hence this influences the market for viral safety practices. Biological products are vulnerable to bacteria, fungi, and viruses. Raw products and improper treatment of culture cause pollution. Viral clearance is an essential process during downstream purification of therapeutic biologics, blood, and tissue-derived materials. They are either removed or inactivated by viruses for manufacturing microbiologically stable goods.

Growth in demand in pharmaceutical and biotechnological industries is the key factor providing viral clearance market opportunity. Moreover, the market development is also propelled by increase in number of new drug releases in different segments and related drug approval procedures.
Viral contamination is a common threat to all animal- and human-derived biopharmaceuticals. This type of contamination can affect any part of a bioproduction process, so bio manufacturers need to perform viral testing studies and incorporate viral clearance methods into their processes. No single test can detect all viruses, and all methods require a minimum level of viruses to be detected. Some methods measure markers of infection, providing results long after infection occurs. Hence, ensuring that a product is free from viral contamination depends on ensuring that a process can inactivate or remove viruses (through viral clearance validation studies) as well as testing for their presence. Such an approach is the only way to ensure that biopharmaceuticals will be free from viral contamination and safe for human use.
Furthermore, the number of new drugs and government policies are witnessing an increase which will further aid growth of viral clearance market size. However, growth of the global virus clearance industry is greatly enhanced by quality assurance and quality management departments. However, high degree of consolidation act is going to restrain the growth of the viral clearance service market.
Increase in number of mergers and acquisitions is anticipated to boost viral clearance market growth in the coming years. For instance, on September 27, 2019, Merck KGaA joined with Pfizer Inc. and launched new drug BAVENCIO for removal of virus. Also, on 1st March 2019, Clean Cells joined with NAOBiOS for development of viral vaccines.
The viral clearance market is segmented into Method, Application and End User. On the basis of method, the viral clearance testing industry is divided into viral removal, and viral inactivation. Viral removal is further divided into chromatography, nanofiltration, and precipitation. Viral inactivation is further categorized into low pH, solvent detergent method, heat pasteurization, and other viral inactivation methods. By application, it is divided into recombinant proteins, blood and blood products, cellular and gene therapy products, vaccines, and other applications. By end user, it is categorized into pharmaceutical & biotechnology companies, contract research organizations, academic research institutes, and others. Region wise, it is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Segment review
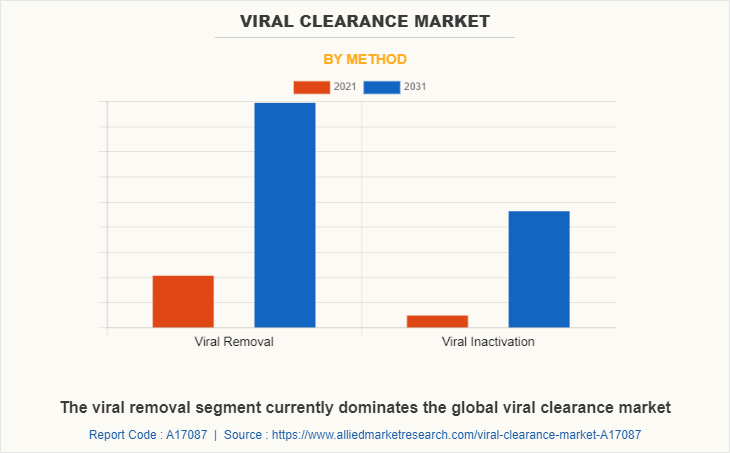
By method, the viral removal segment currently dominates the global viral clearance market share and is expected to continue grow at highest viral clearance market forecast period, owing to increasing awareness about the benefits of using viral removals among healthcare professionals and consumers fueling the demand for viral removals.
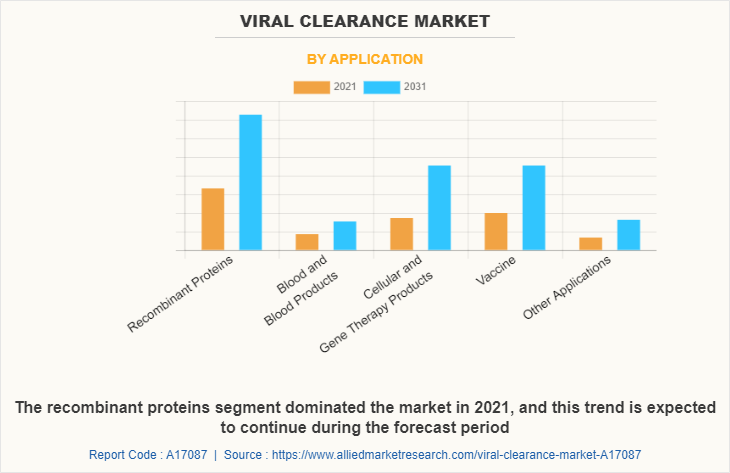
By application, the recombinant proteins segment dominated the market in 2021, and this trend is expected to continue during the forecast period owing to continuous rise of chronic illnesses across the globe. Apart from the surge in chronic diseases, the rise in funding of the worldwide government on R&D activities is also fuelling the market growth. However, the cellular and gene therapy products segment is expected to witness considerable growth during the forecast period due to increase in number of clinical trials of cellular and gene therapy products and increasing investments of pharmaceutical companies in the development of novel drugs is fueling the viral clearance services industry.
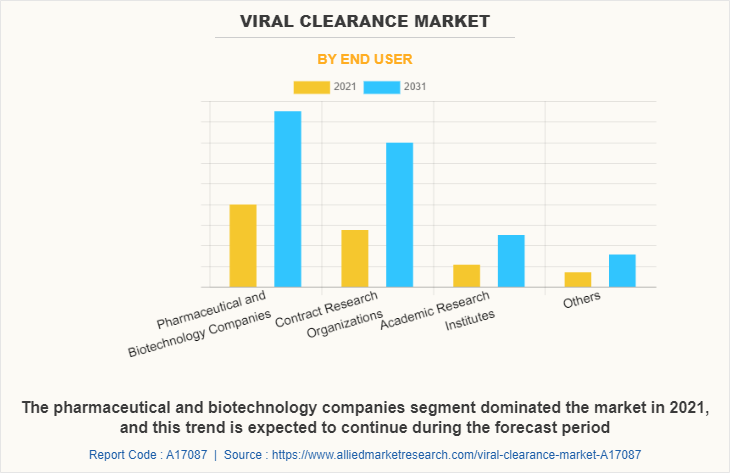
By end user, the pharmaceutical and biotechnology companies segment dominated the market in 2021, and this trend is expected to continue during the forecast period owing to increase in utilization of viral clearance processing during production of biopharmaceuticals, and key players operating in the market are adopting various key strategies to expand their business and rising investment on R&D segment. However, the contract research organizations segment is expected to witness considerable growth during the forecast period due to increasing number of drug manufacturers outsourcing their product testing studies.
Region wise, North America dominated the market in 2021, owing to increase in demand for biopharmaceutical and upsurge in demand for advanced technologies in the purification of recombinant proteins produced by animal cell cultures to create high-value products of modern biotechnology. However, Asia-Pacific is expected to witness considerable market growth during the forecast period due to growing R&D activities for biopharmaceuticals, rise in investments made by the market players and increase in government support. In addition, factors such as increasing skilled workforce and growing biopharmaceutical industry are also supporting the growth of the market in this region.
The major companies profiled in this report include Allure Medical Group, Charles River Laboratories International, Inc., Clean Cells, Creative Biogene, Eurofins Scientific SE, Maravai Lifesciences Holding, Inc., Merck KGaA, Sartorius Stedium Biotech, Syngene International Limited, and Wuxi Biologics Inc.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the viral clearance market analysis from 2021 to 2031 to identify the prevailing viral clearance market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the viral clearance market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global viral clearance market trends, key players, market segments, application areas, and market growth strategies.
Viral clearance Market Report Highlights
| Aspects | Details |
| By Method |
|
| By Application |
|
| By End User |
|
| By Region |
|
| Key Market Players | MARAVAI LIFESCIENCES HOLDINGS, INC., CLEAN CELLS, Charles River Laboratories International, Inc., SYNGENE INTERNATIONAL LIMITED, CREATIVE BIOGENE, EUROFINS SCIENTIFIC SE, ALLURE MEDICAL GROUP, SARTORIUS STEDIM BIOTECH, MERCK KGAA, WUXI BIOLOGICS INC. |
Analyst Review
This section provides the opinions of top level CXOs in the viral clearance market. According to them, viral clearance is used to demonstrate clearance of viruses known to be related to a process. Some methods of viral clearance measure markers of infection, providing results long after infection occurs. Hence, ensuring that a product is free from viral contamination depends on ensuring that a process can inactivate or remove viruses as well as testing for their presence.
As per the analysts, rise in incidence of chronic diseases, technological advancements, and increase in demand of biopharmaceuticals are some of the major factors that drive this market. In addition, increase in prevalence of chronic diseases such as cancer, genetic disorders, and rare diseases among others, has increased the demand for vaccines and antibodies production, which is expected to drive the growth of the market in upcoming years. However, the high cost associated with the virus clearing process and the -consuming process for developing the drug hampers the market growth.
Furthermore, by region, North America is expected to remain dominant during the forecast period, due to local presence of large in-house biopharmaceutical manufacturing facilities. Asia-Pacific registered highest CAGR and is expected to continue this trend throughout the forecast period, owing to rise in expenditure on healthcare & improvement in the medical and healthcare infrastructure.
Allure Medical Group, Charles River Laboratories International, Inc., Clean Cells, Creative Biogene, Eurofins Scientific SE, Maravai Lifesciences Holding, Inc., Merck KGaA, Sartorius Stedium Biotech, Syngene International Limited, and Wuxi Biologics Inc. are the top companies to hold the market share in Viral clearance
Rise in drug launches, increase in R&D investments, and recent advancements in nano-filtration technology are upcoming trends of Viral clearance Market in the world.
Recombinant proteins is the leading application of Viral clearance Market
North America is the largest regional market for Viral clearance
The global viral clearance market was valued at $425.93 million in 2021, and is projected to reach $977.84 million by 2031
Loading Table Of Content...



